当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism of 4-Chlorobiphenyl and the NO3 Radical: An Experimental and Theoretical Study
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-04-26 00:00:00 , DOI: 10.1021/acs.jpca.6b08626 Jin Shi 1 , Wenlong Bi 1 , Shenmin Li 2 , Wenbo Dong 1 , Jianmin Chen 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-04-26 00:00:00 , DOI: 10.1021/acs.jpca.6b08626 Jin Shi 1 , Wenlong Bi 1 , Shenmin Li 2 , Wenbo Dong 1 , Jianmin Chen 1
Affiliation
|
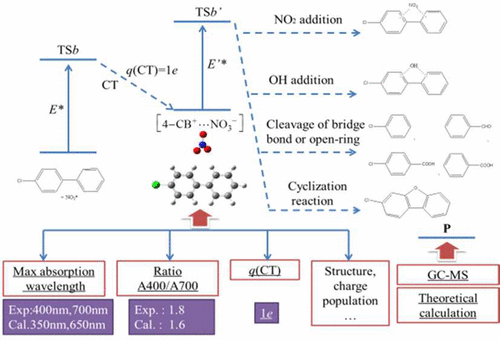
Experiment and theoretical chemistry calculations were conducted to elucidate the mechanism of the reaction between 4-chlorobiphenyl (4-CB) and the NO3 radical. The degradation of PCBs was investigated mechanistically through transient absorption spectroscopy technology and high-accuracy theoretical calculation by using 4-CB as the model. Laser flash photolysis (LFP) experiments were performed at 355 nm. The main intermediate was analyzed through transient absorption spectroscopy and identified to be a charge transfer complex (CTC). The final products were identified through GC–MS analysis. The ground states and excited states of the reactants were calculated through density functional theory (DFT) method. The absorption bands at 400 and 700 nm show good agreement with the experimental results. The ratio of absorbance at 400 and 700 nm is 1.6, and the experimental value is 1.8. Analysis of the charge population indicated that one unit charge transfer from 4-CB to NO3. The entire reaction process was divided into two phases. In the first phase, the CTC intermediate was formed by electrostatic attraction between 4-CB and the NO3 radical. In the second phase, the most important channel of subsequent reactions is the σ-complex as an intermediate formed by N–C coupling. The final product 4-chloro,2-nitrobiphenyl was generated with the breakage of BC–H and BN–O, and benzene derivatives were formed by other channels.
中文翻译:

4-氯联苯与NO 3自由基的反应机理:实验和理论研究
进行了实验和理论化学计算以阐明4-氯联苯(4-CB)与NO 3之间反应的机理激进的。通过瞬态吸收光谱技术和以4-CB为模型的高精度理论计算,对PCB的降解进行了机理研究。激光闪光光解(LFP)实验在355 nm进行。通过瞬态吸收光谱法分析了主要中间体,并将其鉴定为电荷转移络合物(CTC)。最终产品通过GC-MS分析确定。通过密度泛函理论(DFT)方法计算了反应物的基态和激发态。400和700 nm处的吸收带与实验结果显示出良好的一致性。400和700 nm处的吸光度比为1.6,实验值为1.8。电荷总数的分析表明,一个单位电荷从4-CB转移到NO 3。整个反应过程分为两个阶段。在第一阶段中,CTC中间体是通过4-CB和NO 3自由基之间的静电吸引形成的。在第二阶段,后续反应的最重要通道是σ-络合物,它是由N-C偶联形成的中间体。最终产物4-氯,2-硝基联苯是在B C–H和B N–O断裂的情况下生成的,苯衍生物是通过其他通道形成的。
更新日期:2017-04-26
中文翻译:

4-氯联苯与NO 3自由基的反应机理:实验和理论研究
进行了实验和理论化学计算以阐明4-氯联苯(4-CB)与NO 3之间反应的机理激进的。通过瞬态吸收光谱技术和以4-CB为模型的高精度理论计算,对PCB的降解进行了机理研究。激光闪光光解(LFP)实验在355 nm进行。通过瞬态吸收光谱法分析了主要中间体,并将其鉴定为电荷转移络合物(CTC)。最终产品通过GC-MS分析确定。通过密度泛函理论(DFT)方法计算了反应物的基态和激发态。400和700 nm处的吸收带与实验结果显示出良好的一致性。400和700 nm处的吸光度比为1.6,实验值为1.8。电荷总数的分析表明,一个单位电荷从4-CB转移到NO 3。整个反应过程分为两个阶段。在第一阶段中,CTC中间体是通过4-CB和NO 3自由基之间的静电吸引形成的。在第二阶段,后续反应的最重要通道是σ-络合物,它是由N-C偶联形成的中间体。最终产物4-氯,2-硝基联苯是在B C–H和B N–O断裂的情况下生成的,苯衍生物是通过其他通道形成的。









































 京公网安备 11010802027423号
京公网安备 11010802027423号