当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Barium Hydride-Mediated Nitrogen Transfer and Hydrogenation for Ammonia Synthesis: A Case Study of Cobalt
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-04-24 00:00:00 , DOI: 10.1021/acscatal.7b00284 Wenbo Gao 1, 2 , Peikun Wang 1, 2 , Jianping Guo 1, 3 , Fei Chang 1, 2 , Teng He 1 , Qianru Wang 1, 2 , Guotao Wu 1 , Ping Chen 1, 3, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-04-24 00:00:00 , DOI: 10.1021/acscatal.7b00284 Wenbo Gao 1, 2 , Peikun Wang 1, 2 , Jianping Guo 1, 3 , Fei Chang 1, 2 , Teng He 1 , Qianru Wang 1, 2 , Guotao Wu 1 , Ping Chen 1, 3, 4
Affiliation
|
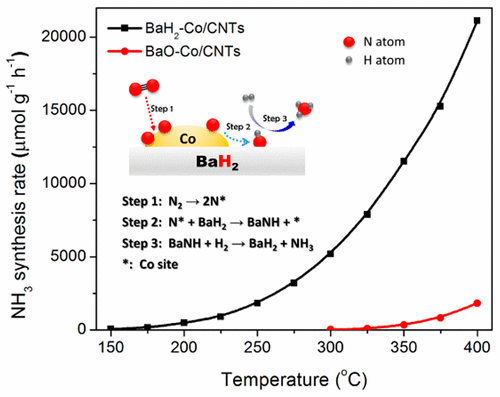
Industrial ammonia synthesis catalyzed by Fe- and Ru-based catalysts is an energy-consuming process. The development of low-temperature active catalyst has been pursued for a century. Herein, we report that barium hydride (BaH2) can synergize with Co, leading to a much better low-temperature activity, i.e., the BaH2-Co/carbon nanotube (CNT) catalyst exhibits ammonia synthesis activity right above 150 °C; at 300 °C, it is 2 orders of magnitude higher than that of the BaO-Co/CNTs and more than 2.5-times higher than Cs-promoted Ru/MgO. Kinetic analyses reveal that the dissociative adsorption of N2 on the Co-BaH2 catalyst may not be the rate-determining step, as evidenced by the much smaller reaction order of N2 (0.43) and the lower apparent activation energy (58 kJ mol–1) compared with those of the unpromoted and BaO-promoted Co-based catalysts. BaH2, with a negative hydride ion, may act as a strong reducing agent, removing activated N from the Co surface and forming a BaNH species. The hydrogenation of the BaNH species to NH3 and BaH2 can be facilely carried out at 150 °C. The relayed catalysis by Co and BaH2 sites creates an energy-favored pathway that allows ammonia synthesis under milder conditions.
中文翻译:

氢化钡介导的氮转移和加氢合成氨的研究:以钴为例
铁和钌基催化剂催化的工业氨合成是一种耗能的过程。低温活性催化剂的开发已经进行了一个世纪。在本文中,我们报道氢化钡(BaH 2)可与Co协同作用,从而导致更好的低温活性,即BaH 2 -Co /碳纳米管(CNT)催化剂在150°C以上具有氨合成活性。在300°C下,它比BaO-Co / CNT高2个数量级,比Cs促进的Ru / MgO高2.5倍以上。动力学分析表明,N 2在Co-BaH 2催化剂上的解离吸附可能不是决定速率的步骤,N 2的反应阶数小得多就证明了这一点。(0.43)和较低的表观活化能(58 kJ mol –1),与未促进和BaO促进的Co基催化剂相比。带有负氢离子的BaH 2可以充当强还原剂,从Co表面去除活化的N并形成BaNH物种。BaNH物质的氢化可容易地在150℃下进行为NH 3和BaH 2。Co和BaH 2位点的中继催化作用产生了能量有利的途径,该途径允许在较温和的条件下合成氨。
更新日期:2017-04-24
中文翻译:

氢化钡介导的氮转移和加氢合成氨的研究:以钴为例
铁和钌基催化剂催化的工业氨合成是一种耗能的过程。低温活性催化剂的开发已经进行了一个世纪。在本文中,我们报道氢化钡(BaH 2)可与Co协同作用,从而导致更好的低温活性,即BaH 2 -Co /碳纳米管(CNT)催化剂在150°C以上具有氨合成活性。在300°C下,它比BaO-Co / CNT高2个数量级,比Cs促进的Ru / MgO高2.5倍以上。动力学分析表明,N 2在Co-BaH 2催化剂上的解离吸附可能不是决定速率的步骤,N 2的反应阶数小得多就证明了这一点。(0.43)和较低的表观活化能(58 kJ mol –1),与未促进和BaO促进的Co基催化剂相比。带有负氢离子的BaH 2可以充当强还原剂,从Co表面去除活化的N并形成BaNH物种。BaNH物质的氢化可容易地在150℃下进行为NH 3和BaH 2。Co和BaH 2位点的中继催化作用产生了能量有利的途径,该途径允许在较温和的条件下合成氨。

































 京公网安备 11010802027423号
京公网安备 11010802027423号