当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Thermal Properties of Difunctional Benzoxazines with Attached Oxazine Ring at the Para-, Meta-, and Ortho-Position
Macromolecules ( IF 5.1 ) Pub Date : 2017-04-24 00:00:00 , DOI: 10.1021/acs.macromol.7b00487 Sini Nalakathu Kolanadiyil 1 , Masaki Minami 2 , Takeshi Endo 1
Macromolecules ( IF 5.1 ) Pub Date : 2017-04-24 00:00:00 , DOI: 10.1021/acs.macromol.7b00487 Sini Nalakathu Kolanadiyil 1 , Masaki Minami 2 , Takeshi Endo 1
Affiliation
|
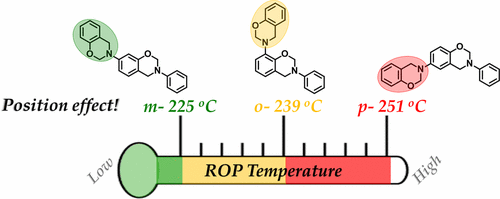
To investigate the role of position of oxazine ring in the benzoxazine backbone on their ring-opening polymerization (ROP) and thermal stability of resulting polybenzoxazine, we have synthesized difunctional monomers solely containing benzoxazine moieties (BZ2) with attached oxazine ring at the para-, meta-, and ortho-position. The ROP was examined by DSC analysis, which revealed a reduction in curing temperature in the order of meta (225 °C) < ortho (239 °C) < para (251 °C). The differences in structural and geometrical parameters were investigated by NMR (1H, 13C, 1H–1H NOESY) and X-ray crystallography analysis. The electronic effects and the intramolecular interaction between oxazine ring and aromatic hydrogen were higher when the attached oxazine ring was at the meta-position. The differences in their positioning also changed their ROP mechanism, an unusual intramolecular polymerization was observed in meta, while in ortho and para the polymerization proceeded in a regular manner. A curing mechanism responsible for lower curing temperature and faster ROP in meta has been proposed, which involves an intramolecular electrophilic substitution of iminium ion, resulting in aza-cyclic rings along with classical phenolic Mannich bridges in the networked structure. The cured resin showed a high Tg and in the order of para (291 °C) > meta (270 °C) > ortho (266 °C). Even though meta-PBZ2 displayed an earlier degradation with Td10 of 358 °C as compared to para and ortho (Td10: 373 °C) due to aza-cyclic rings, the main backbone degradation was observed to be coinciding in all PBZ2s at 417 °C with a char yield of 57% at 600 °C. Thus, changing position of oxazine ring to “meta” in the backbone is a beneficial strategy to have a low curing benzoxazine without sacrificing the thermal stability of resulting polybenzoxazine.
中文翻译:

合成与附恶嗪环的双官能苯并恶嗪的热性能帕拉- ,元-和邻-位置
为了研究恶嗪环在苯并恶嗪骨架中的位置对它们的开环聚合(ROP)和所得聚苯并恶嗪的热稳定性的作用,我们合成了仅含苯并恶嗪部分(BZ2)的双官能单体,在对位-连接有恶嗪环元和邻位。通过DSC分析检查了ROP,发现固化温度降低的幅度为间位(225°C)<邻位(239°C)<对位(251°C)。通过NMR(1 H,13 C,1 H– 1H NOESY)和X射线晶体学分析。当连接的恶嗪环位于间位时,恶嗪环与芳族氢之间的电子效应和分子内相互作用较高。它们在位置上的差异也改变了它们的ROP机理,在间位反应中观察到了不寻常的分子内聚合反应,而在邻位和对位聚合反应则以规则的方式进行。固化机理有助于降低固化温度并加快元数据的ROP已经提出了涉及亚胺离子的分子内亲电取代的方法,其导致网状结构中的氮杂环环以及经典的酚类曼尼希桥。固化的树脂显示出较高的T g,且顺序为对位(291°C)>间位(270°C)>邻位(266°C)。即使与对位和邻位(T d10)相比,间-PBZ2在358°C的T d10时显示出更早的降解。:373°C)(由于氮杂环状环),在417°C下观察到所有PBZ2的主要骨架降解同时发生,在600°C下焦炭产率为57%。因此,在主链中将恶嗪环的位置改变为“间位”是具有低固化苯并恶嗪而不牺牲所得聚苯并恶嗪的热稳定性的有益策略。
更新日期:2017-04-24
中文翻译:

合成与附恶嗪环的双官能苯并恶嗪的热性能帕拉- ,元-和邻-位置
为了研究恶嗪环在苯并恶嗪骨架中的位置对它们的开环聚合(ROP)和所得聚苯并恶嗪的热稳定性的作用,我们合成了仅含苯并恶嗪部分(BZ2)的双官能单体,在对位-连接有恶嗪环元和邻位。通过DSC分析检查了ROP,发现固化温度降低的幅度为间位(225°C)<邻位(239°C)<对位(251°C)。通过NMR(1 H,13 C,1 H– 1H NOESY)和X射线晶体学分析。当连接的恶嗪环位于间位时,恶嗪环与芳族氢之间的电子效应和分子内相互作用较高。它们在位置上的差异也改变了它们的ROP机理,在间位反应中观察到了不寻常的分子内聚合反应,而在邻位和对位聚合反应则以规则的方式进行。固化机理有助于降低固化温度并加快元数据的ROP已经提出了涉及亚胺离子的分子内亲电取代的方法,其导致网状结构中的氮杂环环以及经典的酚类曼尼希桥。固化的树脂显示出较高的T g,且顺序为对位(291°C)>间位(270°C)>邻位(266°C)。即使与对位和邻位(T d10)相比,间-PBZ2在358°C的T d10时显示出更早的降解。:373°C)(由于氮杂环状环),在417°C下观察到所有PBZ2的主要骨架降解同时发生,在600°C下焦炭产率为57%。因此,在主链中将恶嗪环的位置改变为“间位”是具有低固化苯并恶嗪而不牺牲所得聚苯并恶嗪的热稳定性的有益策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号