Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-04-05 , DOI: 10.1016/j.tetlet.2017.04.017 Galina V. Grishina , Ivan S. Veselov , Ekaterina N. Safronova , Dmitriy M. Mazur , Vyacheslav V. Samoshin
|
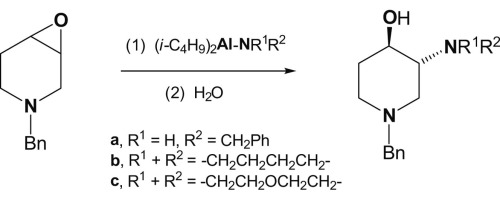
The report presents a first example of a regio- and stereospecific Lewis acid-catalyzed aminolysis of 1-benzyl-3,4-epoxypiperidine leading to trans-3-amino-1-benzylpiperidin-4-ols, in contrast with other Lewis acid-catalyzed reactions leading to trans-4-amino-1-benzylpiperidin-3-ols. The reaction is performed at room temperature using the reagents prepared by interaction of a hard Lewis acid – diisobutylaluminum hydride (DIBAL-H) with primary and secondary amines. The obtained products are potential intermediates on the way to stereochemical analogues of the antitumor piperidine alkaloid pseudodistomin D.
中文翻译:

使用1-二甲基-3,4-环氧哌啶与二异丁基铝酰胺(DIBAL-NR 1 R 2)的区域特异性裂解,可方便地合成反式-3-氨基-1-苄基哌啶-4-醇
该报告提供了第一个例子,与其他路易斯酸-相比,区域和立体特异性路易斯酸催化1-苄基-3,4-环氧哌啶的氨解导致反式-3-氨基-1-苄基哌啶-4-醇的氨解。催化反应,导致反式-4-氨基-1-苄基哌啶-3-醇。反应是在室温下使用由硬路易斯酸–二异丁基氢化铝(DIBAL-H)与伯胺和仲胺相互作用制得的试剂进行的。所获得的产物是抗肿瘤哌啶生物碱假distomin D的立体化学类似物上的潜在中间体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号