Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Chemistry and Humidity in Powder Electrostatics: A Comparative Study between Tribocharging and Corona Discharge
ACS Omega ( IF 3.7 ) Pub Date : 2017-04-21 00:00:00 , DOI: 10.1021/acsomega.7b00125 Karolina W. Biegaj 1 , Martin G. Rowland 2 , Tim M. Lukas 2 , Jerry Y. Y. Heng 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-04-21 00:00:00 , DOI: 10.1021/acsomega.7b00125 Karolina W. Biegaj 1 , Martin G. Rowland 2 , Tim M. Lukas 2 , Jerry Y. Y. Heng 1
Affiliation
|
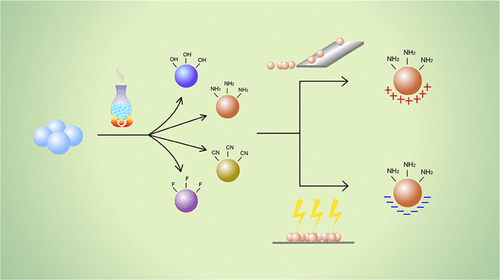
In the present study, the correlation between surface chemical groups and the electrostatic properties of particulate materials was studied. Glass beads were modified to produce OH-, NH2-, CN-, and F-functionalized materials. The materials were charged separately both by friction and by conventional corona charging, and the results were compared. The results obtained from both methods indicated that the electrostatic properties are directly related to the surface functional group chemistry, with hydrophobic groups accumulating greater quantities of charge than hydrophilic groups. The fluorine-rich surface accumulated 5.89 times greater charge upon tribocharging with stainless steel than the hydroxyl-rich surface. However, in contrast to the tribocharging method, the charge polarity could not be determined when corona charging was used. Moreover, discharge profiles at different humidity levels (25% RH, 50% RH, and 75% RH) were obtained for each modified surface, which showed that higher humidity facilitates faster charge decay; however, this enhancement is surface chemistry-dependent. By increasing the humidity from 25% RH to 75% RH, the charge relaxation times can be accelerated 1.6 times for fluorine and 12.2 times for the cyano group. These data confirm that surface functional groups may dictate powder electrostatic behavior and account for observed charge accumulation and discharge phenomena.
中文翻译:

粉末静电中的表面化学和湿度:摩擦充电和电晕放电的比较研究
在本研究中,研究了表面化学基团与颗粒材料静电性能之间的相关性。修饰玻璃珠以产生OH-,NH 2-,CN-和F功能化的材料。通过摩擦和常规电晕充电分别给材料充电,并对结果进行比较。从两种方法获得的结果表明,静电性质与表面官能团的化学性质直接相关,疏水性基团比亲水性基团积累更多的电荷。用不锈钢摩擦充电时,富氟表面的电荷是富羟基表面的5.89倍。但是,与摩擦充电方法相反,当使用电晕充电时无法确定电荷极性。此外,对于每个改性表面,都获得了在不同湿度水平(25%RH,50%RH和75%RH)下的放电曲线,这表明较高的湿度有助于更快的电荷衰减。但是,这种增强是表面化学依赖性的。通过将湿度从25%RH增加到75%RH,对于氟,电荷弛豫时间可以提高1.6倍,对于氰基,则可以提高12.2倍。这些数据证实表面官能团可能决定粉末的静电行为,并解释了观察到的电荷积累和放电现象。
更新日期:2017-04-21
中文翻译:

粉末静电中的表面化学和湿度:摩擦充电和电晕放电的比较研究
在本研究中,研究了表面化学基团与颗粒材料静电性能之间的相关性。修饰玻璃珠以产生OH-,NH 2-,CN-和F功能化的材料。通过摩擦和常规电晕充电分别给材料充电,并对结果进行比较。从两种方法获得的结果表明,静电性质与表面官能团的化学性质直接相关,疏水性基团比亲水性基团积累更多的电荷。用不锈钢摩擦充电时,富氟表面的电荷是富羟基表面的5.89倍。但是,与摩擦充电方法相反,当使用电晕充电时无法确定电荷极性。此外,对于每个改性表面,都获得了在不同湿度水平(25%RH,50%RH和75%RH)下的放电曲线,这表明较高的湿度有助于更快的电荷衰减。但是,这种增强是表面化学依赖性的。通过将湿度从25%RH增加到75%RH,对于氟,电荷弛豫时间可以提高1.6倍,对于氰基,则可以提高12.2倍。这些数据证实表面官能团可能决定粉末的静电行为,并解释了观察到的电荷积累和放电现象。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号