当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Modeling of the Thermal Destruction of Nitrogen Mustard Gas
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-04-20 00:00:00 , DOI: 10.1021/acs.jpca.7b01238
Juan-Carlos Lizardo-Huerta 1 , Baptiste Sirjean 1 , Laurent Verdier 2 , René Fournet 1 , Pierre-Alexandre Glaude 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-04-20 00:00:00 , DOI: 10.1021/acs.jpca.7b01238
Juan-Carlos Lizardo-Huerta 1 , Baptiste Sirjean 1 , Laurent Verdier 2 , René Fournet 1 , Pierre-Alexandre Glaude 1
Affiliation
|
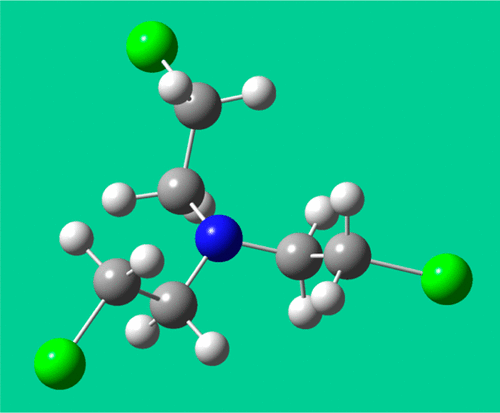
The destruction of stockpiles or unexploded ammunitions of nitrogen mustard (tris(2-chloroethyl)amine, HN-3) requires the development of safe processes. The thermal destruction of this kind of compound is one of the most efficient method of destruction. Because of the high-level of toxicity of this chemical, there is a considerable lack of knowledge on the chemical kinetics at high temperatures. In this study, a detailed chemical kinetic model for the pyrolysis of nitrogen mustard gas is developed based on a large number of thermokinetic parameters calculated with theoretical chemistry. The thermal decomposition of HN-3 is shown to mainly proceed through stepwise dechlorination with Cl-atom being the principal chain carrier. The successive losses of chlorine atom mainly lead to unsaturated amines without chlorine groups. Theoretical calculations demonstrated that the thermal decomposition of these compounds ultimately lead to the formation of pyrrole, which can accumulate at low temperature. At higher temperatures, pyrrole yields HCN and acetylene. Simulations also predict that about 52% of the total flux of decomposition of HN-3 leads to the formation of N,N-diethenyl-2-chloroethylamine (P29), which acts as a chain branching agent because its unimolecular decomposition is preponderant and produces one chlorine and one hydrogen atoms. Comparisons with the simulated reactivity of sulfur mustard gas are also performed and show that HN-3 is more reactive that the former toxic. The higher number of chlorine atoms in HN-3 compared to sulfur mustard (3 vs 2) and the formation of the chain branching intermediate P29 during its decomposition explain this behavior.
中文翻译:

氮芥子气热分解的动力学模型
销毁氮芥(三(2-氯乙基)胺,HN-3)的库存或未爆炸弹药需要开发安全的工艺。这种化合物的热破坏是最有效的破坏方法之一。由于该化学品的高毒性,因此对高温下的化学动力学知识缺乏足够的了解。在这项研究中,基于理论化学计算的大量热动力学参数,开发了氮芥子气热解的详细化学动力学模型。已显示出HN-3的热分解主要是通过逐步脱氯进行的,其中Cl原子为主要链载体。氯原子的连续损失主要导致不具有氯基的不饱和胺。理论计算表明,这些化合物的热分解最终导致吡咯的形成,而吡咯可能在低温下积累。在较高的温度下,吡咯生成HCN和乙炔。模拟还预测,HN-3分解总通量的约52%会导致HN-3的形成。N,N-二乙烯基-2-氯乙胺(P29),由于其单分子分解占优势,并产生一个氯和一个氢原子,因此起链支化剂的作用。还与硫芥子气的模拟反应性进行了比较,结果表明,HN-3的反应性比前者的毒性更高。与硫芥子气相比,HN-3中的氯原子数量更高(3对2),并且在分解过程中形成链支化中间体P29可以解释这种现象。
更新日期:2017-04-20
中文翻译:

氮芥子气热分解的动力学模型
销毁氮芥(三(2-氯乙基)胺,HN-3)的库存或未爆炸弹药需要开发安全的工艺。这种化合物的热破坏是最有效的破坏方法之一。由于该化学品的高毒性,因此对高温下的化学动力学知识缺乏足够的了解。在这项研究中,基于理论化学计算的大量热动力学参数,开发了氮芥子气热解的详细化学动力学模型。已显示出HN-3的热分解主要是通过逐步脱氯进行的,其中Cl原子为主要链载体。氯原子的连续损失主要导致不具有氯基的不饱和胺。理论计算表明,这些化合物的热分解最终导致吡咯的形成,而吡咯可能在低温下积累。在较高的温度下,吡咯生成HCN和乙炔。模拟还预测,HN-3分解总通量的约52%会导致HN-3的形成。N,N-二乙烯基-2-氯乙胺(P29),由于其单分子分解占优势,并产生一个氯和一个氢原子,因此起链支化剂的作用。还与硫芥子气的模拟反应性进行了比较,结果表明,HN-3的反应性比前者的毒性更高。与硫芥子气相比,HN-3中的氯原子数量更高(3对2),并且在分解过程中形成链支化中间体P29可以解释这种现象。































 京公网安备 11010802027423号
京公网安备 11010802027423号