当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu(II)-catalyzed enantioselective 1,3-dipolar cycloaddition of nitrones with α, β-unsaturated acyl phosphonates
Tetrahedron ( IF 2.1 ) Pub Date : 2017-04-21 07:21:21
Lei Xie, Hui Bai, Jiaqi Li, Xuan Yu, Zhenhua Zhang, Bin Fu
Tetrahedron ( IF 2.1 ) Pub Date : 2017-04-21 07:21:21
Lei Xie, Hui Bai, Jiaqi Li, Xuan Yu, Zhenhua Zhang, Bin Fu
|
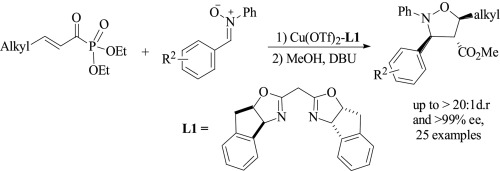
A highly enantioselective 1, 3-dipolar cycloaddition of nitrone with α, β-unsaturated acyl phosphonate was developed for the first time by using a chiral indane-bis(oxazoline)-copper(II) complex. The reaction proceeded smoothly under mild conditions to provide isoxazolidines with multi-stereocenters in good yields with high to excellent diastereo- (>20:1 dr) and enantioselectivities (up to 99% ee). The resulting products were readily converted to multi-functional isoxazolidines or γ-amino alcohol compounds.
中文翻译:

Cu(II)催化的硝基化合物与α,β-不饱和酰基膦酸酯的对映选择性1,3-偶极环加成
通过使用手性茚满-双(恶唑啉)-铜(II)配合物,首次开发了具有α,β-不饱和酰基膦酸酯的高对映选择性的1,3-偶极环硝基加成环。反应在温和条件下平稳进行,以高产率提供具有高至优异的非对映体-(> 20∶1dr)和对映选择性(高达99%ee)的具有多个立体中心的异恶唑烷。所得产物易于转化为多功能异恶唑烷或γ-氨基醇化合物。
更新日期:2017-04-21
中文翻译:

Cu(II)催化的硝基化合物与α,β-不饱和酰基膦酸酯的对映选择性1,3-偶极环加成
通过使用手性茚满-双(恶唑啉)-铜(II)配合物,首次开发了具有α,β-不饱和酰基膦酸酯的高对映选择性的1,3-偶极环硝基加成环。反应在温和条件下平稳进行,以高产率提供具有高至优异的非对映体-(> 20∶1dr)和对映选择性(高达99%ee)的具有多个立体中心的异恶唑烷。所得产物易于转化为多功能异恶唑烷或γ-氨基醇化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号