当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and evaluation of 1,3,6-trisubstituted-4-oxo-1,4-dihydroquinoline-2-carboxylic acid derivatives as ETA receptor selective antagonists using FRET assay
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-04-20 04:43:51 Nikhil Khadtare, Ralph Stephani, Vijaya Korlipara
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2017-04-20 04:43:51 Nikhil Khadtare, Ralph Stephani, Vijaya Korlipara
|
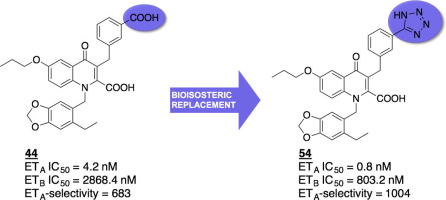
The endothelin axis and in particular the two receptor subtypes, ETA and ETB, are under investigation for the treatment of various diseases such as pulmonary arterial hypertension, fibrosis, renal failure and cancer. Previous work in our lab has shown that 1,3,6-trisubstituted-4-oxo-1,4-dihydroquinoline-2-carboxylic acid derivatives exhibit noteworthy endothelin receptor antagonist activity. A series of analogues with modifications centered around position 6 of the heterocyclic quinolone core and replacement of the aryl carboxylic acid group with an isosteric tetrazole ring was designed and synthesized to further optimize the structure activity relationship. The endothelin receptor antagonist activity was determined by in vitro Förster resonance energy transfer (FRET) using GeneBLAzer® assay technology. The most potent member of this series exhibited ETA receptor antagonist activity in the subnanomolar range with an IC50 value of 0.8 nM, and was 1000-fold selective for the ETA receptor compared to the ETB receptor. Its activity and selectivity profile resembles that of the most recently approved drug, macitentan.
中文翻译:

使用FRET分析法设计,合成和评估1,3,6-三取代-4-氧代-1,4-二氢喹啉-2-羧酸衍生物作为ETA受体选择性拮抗剂
内皮素轴,尤其是两种受体亚型,ET A和ET B,正在研究中,用于治疗各种疾病,例如肺动脉高压,纤维化,肾衰竭和癌症。我们实验室以前的工作表明,1,3,6-三取代-4-氧代-1,4-二氢喹啉-2-羧酸衍生物表现出值得注意的内皮素受体拮抗剂活性。设计并合成了一系列以杂环喹诺酮核的6位为中心进行修饰的类似物,并合成了等位四唑环取代芳基羧酸基团,以进一步优化结构活性关系。使用GeneBLAzer通过体外Förster共振能量转移(FRET)确定了内皮素受体拮抗剂的活性®检测技术。该系列中最有力的成员在亚纳摩尔范围内表现出ET A受体拮抗剂活性,IC 50值为0.8 nM,与ET B受体相比,对ET A受体的选择性是其1000倍。它的活性和选择性与最近批准的药物马西坦坦相似。
更新日期:2017-04-20
中文翻译:

使用FRET分析法设计,合成和评估1,3,6-三取代-4-氧代-1,4-二氢喹啉-2-羧酸衍生物作为ETA受体选择性拮抗剂
内皮素轴,尤其是两种受体亚型,ET A和ET B,正在研究中,用于治疗各种疾病,例如肺动脉高压,纤维化,肾衰竭和癌症。我们实验室以前的工作表明,1,3,6-三取代-4-氧代-1,4-二氢喹啉-2-羧酸衍生物表现出值得注意的内皮素受体拮抗剂活性。设计并合成了一系列以杂环喹诺酮核的6位为中心进行修饰的类似物,并合成了等位四唑环取代芳基羧酸基团,以进一步优化结构活性关系。使用GeneBLAzer通过体外Förster共振能量转移(FRET)确定了内皮素受体拮抗剂的活性®检测技术。该系列中最有力的成员在亚纳摩尔范围内表现出ET A受体拮抗剂活性,IC 50值为0.8 nM,与ET B受体相比,对ET A受体的选择性是其1000倍。它的活性和选择性与最近批准的药物马西坦坦相似。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号