当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
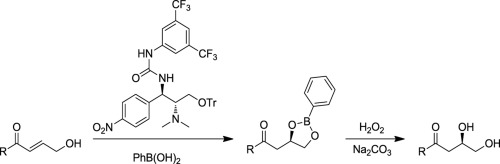
Chloramphenicol base chemistry. Part 101: Asymmetric synthesis of α-hydroxy chiral alcohols via intramolecular Michael additions of γ-hydroxy-α, β-unsaturated enones with chloramphenicol base derived bifunctional urea organocatalysts
Tetrahedron ( IF 2.1 ) Pub Date : 2017-04-12 13:29:35 Haifeng Wang, Linjie Yan, Yan Wu, Fener Chen
Tetrahedron ( IF 2.1 ) Pub Date : 2017-04-12 13:29:35 Haifeng Wang, Linjie Yan, Yan Wu, Fener Chen
|
We have developed the chloramphenicol base urea-catalyzed intramolecular Michael addition of γ-hydroxy-α, β-unsaturated enones. The oxidation of the resulting products provided facile access to the corresponding α-hydroxy chiral alcohols with good efficiency and enantioselectivity, with the reaction displaying broad substrate scope. The utility of this methodology was further demonstrated by the synthesis of (R)-2-hydroxy-4-phenylbutanoate, which is a key building block for the construction of the ACE inhibitor benazepril hydrochloride.
中文翻译:

氯霉素基础化学。第101部分:通过用氯霉素碱衍生的双功能尿素有机催化剂通过分子内迈克尔加成γ-羟基-α,β-不饱和烯酮的不对称合成α-羟基手性醇
我们已经开发了氯霉素碱脲催化的γ-羟基-α,β-不饱和烯酮的分子内迈克尔加成。所得产物的氧化提供了以良好的效率和对映选择性容易地获得相应的α-羟基手性醇的反应,该反应显示出广泛的底物范围。通过合成(R)-2-羟基-4-苯基丁酸(该化合物是构建ACEI抑制剂贝那普利盐酸盐的关键组成部分),进一步证明了该方法的实用性。
更新日期:2017-04-13
中文翻译:

氯霉素基础化学。第101部分:通过用氯霉素碱衍生的双功能尿素有机催化剂通过分子内迈克尔加成γ-羟基-α,β-不饱和烯酮的不对称合成α-羟基手性醇
我们已经开发了氯霉素碱脲催化的γ-羟基-α,β-不饱和烯酮的分子内迈克尔加成。所得产物的氧化提供了以良好的效率和对映选择性容易地获得相应的α-羟基手性醇的反应,该反应显示出广泛的底物范围。通过合成(R)-2-羟基-4-苯基丁酸(该化合物是构建ACEI抑制剂贝那普利盐酸盐的关键组成部分),进一步证明了该方法的实用性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号