当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Recognition-Based DNA Nanoassemblies on the Surfaces of Nanosized Exosomes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-04-07 , DOI: 10.1021/jacs.7b00319 Shuo Wan 1 , Liqin Zhang 1, 2 , Sai Wang 1 , Yuan Liu 1, 2 , Cuichen Wu 1, 2 , Cheng Cui 1 , Hao Sun 1 , Muling Shi 1, 2 , Ying Jiang 1, 2 , Long Li 1 , Liping Qiu 1, 2 , Weihong Tan 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-04-07 , DOI: 10.1021/jacs.7b00319 Shuo Wan 1 , Liqin Zhang 1, 2 , Sai Wang 1 , Yuan Liu 1, 2 , Cuichen Wu 1, 2 , Cheng Cui 1 , Hao Sun 1 , Muling Shi 1, 2 , Ying Jiang 1, 2 , Long Li 1 , Liping Qiu 1, 2 , Weihong Tan 1, 2
Affiliation
|
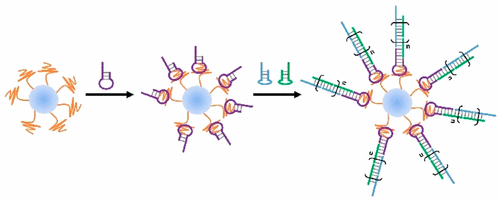
Exosomes are membrane-enclosed extracellular vesicles derived from cells, carrying biomolecules that include proteins and nucleic acids for intercellular communication. Owning to their advantages of size, structure, stability, and biocompatibility, exosomes have been used widely as natural nanocarriers for intracellular delivery of theranostic agents. Meanwhile, surface modifications needed to endow exosomes with additional functionalities remain challenging by their small size and the complexity of their membrane surfaces. Current methods have used genetic engineering and chemical conjugation, but these strategies require complex manipulations and have only limited applications. Herein, we present an aptamer-based DNA nanoassemblies on exosome surfaces. This in situ assembly method is based on molecular recognition between DNA aptamers and their exosome surface markers, as well as DNA hybridization chain reaction initiated by an aptamer-chimeric trigger. It further demonstrated selective assembly on target cell-derived exosomes, but not exosomes derived from nontarget cells. The present work shows that DNA nanostructures can successfully be assembled on a nanosized organelle. This approach is useful for exosome modification and functionalization, which is expected to have broad biomedical and bioanalytical applications.
中文翻译:

纳米外泌体表面基于分子识别的 DNA 纳米组装体
外泌体是源自细胞的膜封闭的细胞外囊泡,携带生物分子,包括用于细胞间通讯的蛋白质和核酸。由于其尺寸、结构、稳定性和生物相容性等优点,外泌体已被广泛用作细胞内递送治疗诊断剂的天然纳米载体。与此同时,由于外泌体尺寸小且膜表面复杂,赋予外泌体额外功能所需的表面修饰仍然具有挑战性。目前的方法使用了基因工程和化学缀合,但这些策略需要复杂的操作,并且应用有限。在此,我们在外泌体表面展示了一种基于适配体的 DNA 纳米组件。这种原位组装方法基于DNA适体与其外泌体表面标记之间的分子识别,以及由适体嵌合触发器引发的DNA杂交链式反应。它进一步证明了对靶细胞来源的外泌体的选择性组装,但对非靶细胞来源的外泌体则不然。目前的工作表明 DNA 纳米结构可以成功地组装在纳米尺寸的细胞器上。这种方法可用于外泌体修饰和功能化,预计将具有广泛的生物医学和生物分析应用。
更新日期:2017-04-07
中文翻译:

纳米外泌体表面基于分子识别的 DNA 纳米组装体
外泌体是源自细胞的膜封闭的细胞外囊泡,携带生物分子,包括用于细胞间通讯的蛋白质和核酸。由于其尺寸、结构、稳定性和生物相容性等优点,外泌体已被广泛用作细胞内递送治疗诊断剂的天然纳米载体。与此同时,由于外泌体尺寸小且膜表面复杂,赋予外泌体额外功能所需的表面修饰仍然具有挑战性。目前的方法使用了基因工程和化学缀合,但这些策略需要复杂的操作,并且应用有限。在此,我们在外泌体表面展示了一种基于适配体的 DNA 纳米组件。这种原位组装方法基于DNA适体与其外泌体表面标记之间的分子识别,以及由适体嵌合触发器引发的DNA杂交链式反应。它进一步证明了对靶细胞来源的外泌体的选择性组装,但对非靶细胞来源的外泌体则不然。目前的工作表明 DNA 纳米结构可以成功地组装在纳米尺寸的细胞器上。这种方法可用于外泌体修饰和功能化,预计将具有广泛的生物医学和生物分析应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号