当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of highly functionalized 1,5-disubstituted tetrazoles via palladium-catalyzed Suzuki coupling
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-04-02 15:09:44 Edward J. Hennessy, Mark Cornebise, Lakshmaiah Gingipalli, Tyler Grebe, Sudhir Hande, Valerie Hoesch, Hoan Huynh, Scott Throner, Jeffrey Varnes, Ye Wu
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-04-02 15:09:44 Edward J. Hennessy, Mark Cornebise, Lakshmaiah Gingipalli, Tyler Grebe, Sudhir Hande, Valerie Hoesch, Hoan Huynh, Scott Throner, Jeffrey Varnes, Ye Wu
|
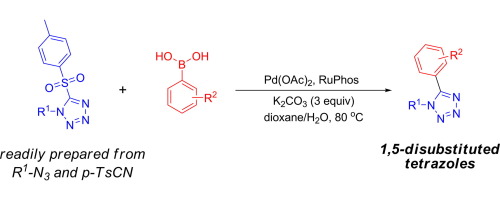
The preparation of a range of 1,5-disubstituted tetrazoles has been achieved through palladium-catalyzed Suzuki coupling. Using appropriately substituted 5-p-toluenesulfonyltetrazoles as substrates (obtained by cycloaddition of a substituted azide with p-toluenesulfonyl cyanide), this methodology provides access to a variety of highly substituted tetrazoles that would be difficult to access otherwise. The procedure is compatible with functional groups commonly found in drug-like molecules, and has been used to generate a number of compounds of potential biological interest.
中文翻译:

通过钯催化的Suzuki偶联制备高度官能化的1,5-二取代的四唑
通过钯催化的Suzuki偶联已经实现了一系列1,5-二取代的四唑的制备。使用适当取代的5-对甲苯磺酰基四唑作为底物(通过将取代的叠氮化物与对甲苯磺酰基氰基环加成而获得),该方法提供了多种否则难以获得的高度取代的四唑的访问。该方法与通常在药物样分子中发现的官能团兼容,并已用于产生许多具有潜在生物学意义的化合物。
更新日期:2017-04-03
中文翻译:

通过钯催化的Suzuki偶联制备高度官能化的1,5-二取代的四唑
通过钯催化的Suzuki偶联已经实现了一系列1,5-二取代的四唑的制备。使用适当取代的5-对甲苯磺酰基四唑作为底物(通过将取代的叠氮化物与对甲苯磺酰基氰基环加成而获得),该方法提供了多种否则难以获得的高度取代的四唑的访问。该方法与通常在药物样分子中发现的官能团兼容,并已用于产生许多具有潜在生物学意义的化合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号