当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diisobutylaluminum hydride-promoted cyclization of silylated 1,3-dien-5-ynes: Application to total synthesis of a 20-norabietane derivative
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-03-31 13:33:08 Hidenori Kinoshita, Kazuki Yaguchi, Takayuki Tohjima, Katsukiyo Miura
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2017-03-31 13:33:08 Hidenori Kinoshita, Kazuki Yaguchi, Takayuki Tohjima, Katsukiyo Miura
|
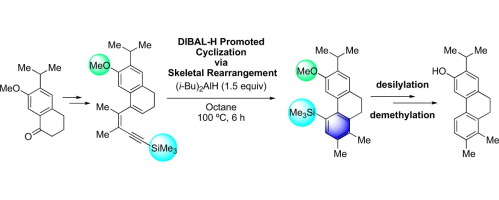
The diisobutylaluminum hydride (DIBAL-H)-promoted benzocyclization, recently developed by this group, was adopted for the synthesis of a natural product containing a 9,10-dihydrophenanthrene skeleton to demonstrate its synthetic utility. One of the extracts from the roots of Salvia hydrangea DC. ex Bentham (Lamiaceae), a 20-norabietane derivative, was selected as the target molecule. The key step forming the 9,10-dihydrophenanthrene skeleton was achieved by the DIBAL-H-promoted cyclization of a silylated 1,3-dien-5-yne easily accessible from a substituted α-tetralone.
中文翻译:

二异丁基氢化铝促进的甲硅烷基化1,3-二烯-5-炔的环化反应:在全合成20-壬二烷衍生物中的应用
该小组最近开发的由二异丁基氢化铝(DIBAL-H)促进的苯并环化反应被用于合成含有9,10-二氢菲骨架的天然产物,以证明其合成用途。丹参绣球DC的根中的一种提取物。选择一种20-norabietane衍生物的前Bentham(唇形科)作为目标分子。形成9,10-二氢菲骨架的关键步骤是通过DIBAL-H促进的硅烷化1,3-二烯-5-炔基的环化反应而实现的,该环化反应可轻松地从取代的α-四氢萘酮中获得。
更新日期:2017-04-01
中文翻译:

二异丁基氢化铝促进的甲硅烷基化1,3-二烯-5-炔的环化反应:在全合成20-壬二烷衍生物中的应用
该小组最近开发的由二异丁基氢化铝(DIBAL-H)促进的苯并环化反应被用于合成含有9,10-二氢菲骨架的天然产物,以证明其合成用途。丹参绣球DC的根中的一种提取物。选择一种20-norabietane衍生物的前Bentham(唇形科)作为目标分子。形成9,10-二氢菲骨架的关键步骤是通过DIBAL-H促进的硅烷化1,3-二烯-5-炔基的环化反应而实现的,该环化反应可轻松地从取代的α-四氢萘酮中获得。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号