当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective and Efficient Removal of Toxic Oxoanions of As(III), As(V), and Cr(VI) by Layered Double Hydroxide Intercalated with MoS42–
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-03-30 00:00:00 , DOI: 10.1021/acs.chemmater.7b00618 Lijiao Ma 1 , Saiful M. Islam 2 , Hongyun Liu 1 , Jing Zhao 2 , Genban Sun 1 , Huifeng Li 1 , Shulan Ma 1, 2 , Mercouri G. Kanatzidis 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-03-30 00:00:00 , DOI: 10.1021/acs.chemmater.7b00618 Lijiao Ma 1 , Saiful M. Islam 2 , Hongyun Liu 1 , Jing Zhao 2 , Genban Sun 1 , Huifeng Li 1 , Shulan Ma 1, 2 , Mercouri G. Kanatzidis 2
Affiliation
|
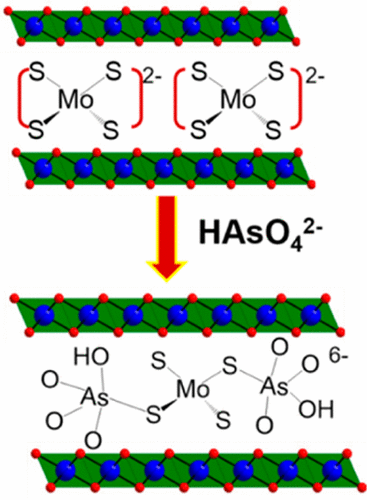
We demonstrate the highly selective and exceptionally efficient capture of toxic oxoanions of As(III)/As(V) (HAsO32–/HAsO42–) and Cr(VI) (CrO42–) by the Mg/Al layered double hydroxide (Mg/Al-LDH) intercalated with MoS42– (MoS4-LDH). The MoS4-LDH exhibits very high removal rates (>99%) of As(III), As(V), and Cr(VI) from complex aqueous solutions. In the presence of many competing nontoxic anions such as SO42–, NO3–, and Cl–, remarkable selectivity for HAsO32–, HAsO42–, and CrO42– is observed. Highly concentrated As(III) and Cr(VI) can be rapidly reduced to <10 ppb, much below the permitted level for drinking water. The maximum adsorption capacities for As(III), As(V), and Cr(VI) are 99, 56, and 130 mg/g, respectively. Sorption isotherms for As(III) and As(V) agree with the Langmuir model, suggesting a monolayer adsorption on the sorbent. The adsorptions of As(V) and Cr(VI) are exceptionally rapid, showing >93% removals within 1 min and >96% removal within 5 min. Sorption kinetics for these oxoanions follows a pseudo-second-order model consistent with a chemisorption involving possible As–S and Cr–S bonding. The Cr(VI) adsorption is accompanied by an oxidation/reduction reaction with MoS42– resulting in Cr(III) species. The order of rate constants (k2) is Cr(VI) > As(V) > As(III), which reflects their respective adsorption rates. The S–As and S–Cr interactions and the reduction of Cr(VI) to Cr(III) contribute to the effective uptakes. These results make the MoS4-LDH material a compelling candidate for application in remediation of water polluted with arsenic and hexavalent chromium.
中文翻译:

嵌入MoS 4 2的层状双氢氧化物选择性高效去除As(III),As(V)和Cr(VI)的有毒氧阴离子
我们证明了通过层状Mg / Al可以高度选择性且异常有效地捕获As(III)/ As(V)(HAsO 3 2– / HAsO 4 2–)和Cr(VI)(CrO 4 2–)的有毒氧阴离子双氢氧化物(Mg / Al-LDH)嵌入MoS 4 2–(MoS 4 -LDH)。MoS 4 -LDH表现出从复杂水溶液中非常高的As(III),As(V)和Cr(VI)去除率(> 99%)。在许多竞争无毒阴离子如SO存在4 2-,NO 3 -和Cl - ,为HAsO显着的选择性3 2-,HAsO 42 –和CrO 4 2–被观察到。高浓度的As(III)和Cr(VI)可以迅速降低至<10 ppb,远低于饮用水的允许水平。As(III),As(V)和Cr(VI)的最大吸附容量分别为99、56和130 mg / g。As(III)和As(V)的吸附等温线与Langmuir模型一致,表明吸附剂上存在单层吸附。As(V)和Cr(VI)的吸附非常快,在1分钟内显示> 93%的去除,在5分钟内显示> 96%的去除。这些含氧阴离子的吸附动力学遵循伪二级模型,与涉及可能的As-S和Cr-S键的化学吸附相一致。Cr(VI)的吸附伴随着与MoS 4 2–的氧化/还原反应产生Cr(III)物质。速率常数(k 2)的顺序为Cr(VI)> As(V)> As(III),反映了它们各自的吸附速率。S–As和S–Cr相互作用以及Cr(VI)还原为Cr(III)有助于有效吸收。这些结果使MoS 4 -LDH材料成为用于修复被砷和六价铬污染的水的引人注目的候选材料。
更新日期:2017-03-30
中文翻译:

嵌入MoS 4 2的层状双氢氧化物选择性高效去除As(III),As(V)和Cr(VI)的有毒氧阴离子
我们证明了通过层状Mg / Al可以高度选择性且异常有效地捕获As(III)/ As(V)(HAsO 3 2– / HAsO 4 2–)和Cr(VI)(CrO 4 2–)的有毒氧阴离子双氢氧化物(Mg / Al-LDH)嵌入MoS 4 2–(MoS 4 -LDH)。MoS 4 -LDH表现出从复杂水溶液中非常高的As(III),As(V)和Cr(VI)去除率(> 99%)。在许多竞争无毒阴离子如SO存在4 2-,NO 3 -和Cl - ,为HAsO显着的选择性3 2-,HAsO 42 –和CrO 4 2–被观察到。高浓度的As(III)和Cr(VI)可以迅速降低至<10 ppb,远低于饮用水的允许水平。As(III),As(V)和Cr(VI)的最大吸附容量分别为99、56和130 mg / g。As(III)和As(V)的吸附等温线与Langmuir模型一致,表明吸附剂上存在单层吸附。As(V)和Cr(VI)的吸附非常快,在1分钟内显示> 93%的去除,在5分钟内显示> 96%的去除。这些含氧阴离子的吸附动力学遵循伪二级模型,与涉及可能的As-S和Cr-S键的化学吸附相一致。Cr(VI)的吸附伴随着与MoS 4 2–的氧化/还原反应产生Cr(III)物质。速率常数(k 2)的顺序为Cr(VI)> As(V)> As(III),反映了它们各自的吸附速率。S–As和S–Cr相互作用以及Cr(VI)还原为Cr(III)有助于有效吸收。这些结果使MoS 4 -LDH材料成为用于修复被砷和六价铬污染的水的引人注目的候选材料。































 京公网安备 11010802027423号
京公网安备 11010802027423号