当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkene–Carbene Isomerization induced by Borane: Access to an Asymmetrical Diborene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-28 , DOI: 10.1021/jacs.7b02251 Wei Lu 1 , Yongxin Li 1 , Rakesh Ganguly 1 , Rei Kinjo 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-28 , DOI: 10.1021/jacs.7b02251 Wei Lu 1 , Yongxin Li 1 , Rakesh Ganguly 1 , Rei Kinjo 1
Affiliation
|
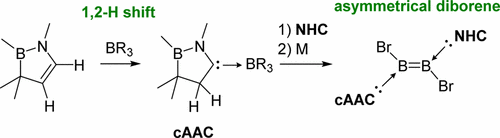
A 2,3-dihydro-1H-1,2-azaborole derivative 2 was converted to a cyclic (alkyl) (amino)carbene (cAAC) via 1,2-hydrogen migration triggered by boranes to afford cAAC-borane adducts. This procedure allowed us to develop an asymmetrical diborene cAAC·(Br)B═B(Br)·IDip 6, which was isolated and fully characterized. The 11B NMR spectrum, X-ray diffraction analysis and computational studies indicate that π-electrons on the central B2 moiety in 6 are unequivalently distributed, and thus polarized. A complete scission of the B═B double bond in 6 was achieved by the treatment with an isonitrile, which led to the formation of a base-stabilized B,N-containing methylenecyclopropane 7.
中文翻译:

硼烷引起的烯烃-卡宾异构化:获得不对称二硼烯
2,3-二氢-1H-1,2-氮杂硼衍生物 2 通过硼烷引发的 1,2-氢迁移转化为环状(烷基)(氨基)卡宾 (cAAC),以提供 cAAC-硼烷加合物。该程序使我们能够开发出不对称的二硼烯 cAAC·(Br)B=B(Br)·IDip 6,它被分离出来并被充分表征。11B NMR 光谱、X 射线衍射分析和计算研究表明,6 中中心 B2 部分上的 π 电子是不等价分布的,因此是极化的。通过用异腈处理实现了 6 中 B=B 双键的完全断裂,这导致形成碱稳定的含 B,N 的亚甲基环丙烷 7。
更新日期:2017-03-28
中文翻译:

硼烷引起的烯烃-卡宾异构化:获得不对称二硼烯
2,3-二氢-1H-1,2-氮杂硼衍生物 2 通过硼烷引发的 1,2-氢迁移转化为环状(烷基)(氨基)卡宾 (cAAC),以提供 cAAC-硼烷加合物。该程序使我们能够开发出不对称的二硼烯 cAAC·(Br)B=B(Br)·IDip 6,它被分离出来并被充分表征。11B NMR 光谱、X 射线衍射分析和计算研究表明,6 中中心 B2 部分上的 π 电子是不等价分布的,因此是极化的。通过用异腈处理实现了 6 中 B=B 双键的完全断裂,这导致形成碱稳定的含 B,N 的亚甲基环丙烷 7。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号