当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,6‐Dihalogenated Purine Nucleosides by Thermostable Nucleoside Phosphorylases
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-03-25 , DOI: 10.1002/adsc.201400966
Xinrui Zhou 1 , Kathleen Szeker 1 , Lin‐Yu Jiao 2 , Martin Oestreich 2 , Igor A. Mikhailopulo 3 , Peter Neubauer 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2015-03-25 , DOI: 10.1002/adsc.201400966
Xinrui Zhou 1 , Kathleen Szeker 1 , Lin‐Yu Jiao 2 , Martin Oestreich 2 , Igor A. Mikhailopulo 3 , Peter Neubauer 1
Affiliation
|
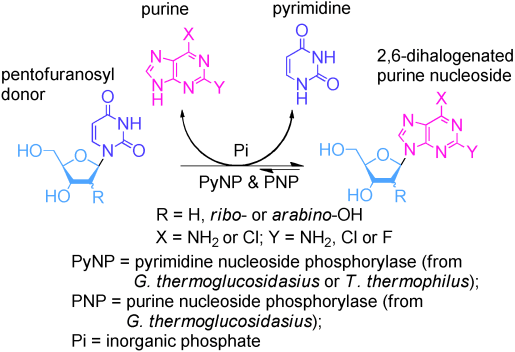
The enzymatic transglycosylation of 2,6‐dichloropurine (26DCP) and 6‐chloro‐2‐fluoropurine (6C2FP) with uridine, thymidine and 1‐(β‐D‐arabinofuranosyl)‐uracil as the pentofuranose donors and recombinant thermostable nucleoside phosphorylases from G. thermoglucosidasius or T. thermophilus as biocatalysts was studied. Selection of 26DCP and 6C2FP as substrates is determined by their higher solubility in aqueous buffer solutions compared to most natural and modified purines and, furthermore, synthesized nucleosides are valuable precursors for the preparation of a large number of biologically important nucleosides. The substrate activity of 26DCP and 6C2FP in the synthesis of their ribo‐ and 2′‐deoxyribo‐nucleosides was closely similar to that of related 2‐amino‐ (DAP), 2‐chloro‐ and 2‐fluoroadenines; the efficiency of the synthesis of β‐D‐arabinofuranosides of 26DCP and 6C2FP was lower vs. that of DAP under similar reaction conditions. For a convenient and easier recovery of the biocatalysts, the thermostable enzymes were immobilized on MagReSyn® epoxide beads and the biocatalyst showed high catalytic efficiency in a number of reactions. As an example, 6‐chloro‐2‐fluoro‐(β‐D‐ribofuranosyl)‐purine (9), a precursor of various antiviral and antitumour drugs, was synthesized by the immobilized enzymes at 60 °C under high substrate concentrations (uridine:purine ratio of 2:1, mol). The synthesis was successfully scaled‐up [uridine (2.5 mmol), base (1.25 mmol); reaction mixture 50 mL] to afford 9 in 60% yield. The reaction reveals the great practical potential of this enzymatic method for the efficient production of modified purine nucleosides of pharmaceutical interest.
中文翻译:

热稳定的核苷磷酸化酶合成2,6-二卤代嘌呤核苷
2,6-二氯嘌呤(26DCP)和6-氯-2-氟嘌呤(6C2FP)与尿苷,胸苷和1-(β- D-阿拉伯呋喃糖基)-尿嘧啶作为戊呋喃糖供体的酶促转糖基化和G的重组热稳定核苷磷酸化酶研究了嗜热葡萄糖假单胞菌或嗜热毁丝霉(T.thermophilus)作为生物催化剂。与大多数天然和修饰的嘌呤相比,26DCP和6C2FP作为底物的选择取决于它们在水性缓冲溶液中的溶解度更高,此外,合成的核苷是制备大量生物学上重要的核苷的有价值的前体。26DCP和6C2FP在核糖和2'-脱氧核糖合成中的底物活性核苷与相关的2-氨基(DAP),2-氯和2-氟ade啶的核苷极为相似。β-的合成效率d 26DCP和6C2FP的-arabinofuranosides较低与类似的反应条件下该DAP的。对于生物催化剂的方便和更容易回收,热稳定酶固定在MagReSyn ®环氧珠和生物催化剂在许多反应显示出高催化效率。例如,6-氯-2-氟-(β- D-核呋喃糖基)-嘌呤(9)是各种抗病毒和抗肿瘤药物的前体,是通过固定化酶在60°C下在高底物浓度(尿苷:嘌呤比为2:1,mol)下合成的。合成成功地放大[尿苷(2.5 mmol),碱(1.25 mmol);反应混合物50毫升],以60%的收率得到9。该反应揭示了该酶法有效生产具有药用价值的修饰的嘌呤核苷的巨大实践潜力。
更新日期:2015-03-25
中文翻译:

热稳定的核苷磷酸化酶合成2,6-二卤代嘌呤核苷
2,6-二氯嘌呤(26DCP)和6-氯-2-氟嘌呤(6C2FP)与尿苷,胸苷和1-(β- D-阿拉伯呋喃糖基)-尿嘧啶作为戊呋喃糖供体的酶促转糖基化和G的重组热稳定核苷磷酸化酶研究了嗜热葡萄糖假单胞菌或嗜热毁丝霉(T.thermophilus)作为生物催化剂。与大多数天然和修饰的嘌呤相比,26DCP和6C2FP作为底物的选择取决于它们在水性缓冲溶液中的溶解度更高,此外,合成的核苷是制备大量生物学上重要的核苷的有价值的前体。26DCP和6C2FP在核糖和2'-脱氧核糖合成中的底物活性核苷与相关的2-氨基(DAP),2-氯和2-氟ade啶的核苷极为相似。β-的合成效率d 26DCP和6C2FP的-arabinofuranosides较低与类似的反应条件下该DAP的。对于生物催化剂的方便和更容易回收,热稳定酶固定在MagReSyn ®环氧珠和生物催化剂在许多反应显示出高催化效率。例如,6-氯-2-氟-(β- D-核呋喃糖基)-嘌呤(9)是各种抗病毒和抗肿瘤药物的前体,是通过固定化酶在60°C下在高底物浓度(尿苷:嘌呤比为2:1,mol)下合成的。合成成功地放大[尿苷(2.5 mmol),碱(1.25 mmol);反应混合物50毫升],以60%的收率得到9。该反应揭示了该酶法有效生产具有药用价值的修饰的嘌呤核苷的巨大实践潜力。







































 京公网安备 11010802027423号
京公网安备 11010802027423号