Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Mechanism of the Dissolution of Quartz and Silica in Aqueous Solutions
ACS Omega ( IF 3.7 ) Pub Date : 2017-03-22 00:00:00 , DOI: 10.1021/acsomega.7b00019 Frank K. Crundwell 1
ACS Omega ( IF 3.7 ) Pub Date : 2017-03-22 00:00:00 , DOI: 10.1021/acsomega.7b00019 Frank K. Crundwell 1
Affiliation
|
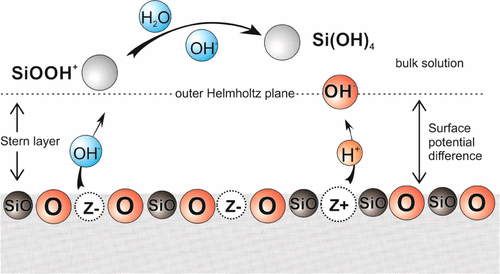
Quartz and silica are common materials, and their dissolution is of significant interest to a wide range of scientists. The kinetics of the dissolution of quartz and silica have been measured extensively, yet no clear theory of dissolution is available. A novel theory of dissolution and crystallization has recently been proposed that envisages the removal of material from the surface to form ions in solution leaving behind a charged surface vacancy. These vacancies create a potential difference across the Stern layer that accelerates or retards the removal of ions. In this way, the surface potential difference is caused by and influences the rate of the removal of ions. From this theory, a model of quartz dissolution is derived that predicts the observed orders of reaction. This prediction of the orders of reaction fits a data set consisting of 285 experiments. The model also describes the effect of Na+, K+, and Li+ ions, as well as the effect of heavy water. A significant component of the model is its ability to describe the zeta potential of the quartz–water interface. The model successfully predicts a transient period at the beginning of the reaction when the rate could either increase or decrease.
中文翻译:

水溶液中石英和二氧化硅的溶解机理
石英和二氧化硅是常见的材料,它们的溶解引起众多科学家的极大兴趣。已经广泛地测量了石英和二氧化硅溶解的动力学,但是尚无明确的溶解理论。最近提出了一种新的溶解和结晶理论,该理论设想从表面除去材料以在溶液中形成离子,从而留下带电的表面空位。这些空位会在整个斯特恩层上产生电势差,从而加速或阻碍离子的去除。这样,表面电势差是由离子的去除速率引起的并且影响离子的去除速率。从该理论出发,推导了石英溶解模型,该模型可预测观察到的反应顺序。反应顺序的这种预测适合由285个实验组成的数据集。该模型还描述了Na的作用+,K +和Li +离子,以及重水的作用。该模型的重要组成部分是它能够描述石英-水界面的ζ电势。该模型可以成功地预测反应开始时的过渡时期,此时速率可以提高或降低。
更新日期:2017-03-22
中文翻译:

水溶液中石英和二氧化硅的溶解机理
石英和二氧化硅是常见的材料,它们的溶解引起众多科学家的极大兴趣。已经广泛地测量了石英和二氧化硅溶解的动力学,但是尚无明确的溶解理论。最近提出了一种新的溶解和结晶理论,该理论设想从表面除去材料以在溶液中形成离子,从而留下带电的表面空位。这些空位会在整个斯特恩层上产生电势差,从而加速或阻碍离子的去除。这样,表面电势差是由离子的去除速率引起的并且影响离子的去除速率。从该理论出发,推导了石英溶解模型,该模型可预测观察到的反应顺序。反应顺序的这种预测适合由285个实验组成的数据集。该模型还描述了Na的作用+,K +和Li +离子,以及重水的作用。该模型的重要组成部分是它能够描述石英-水界面的ζ电势。该模型可以成功地预测反应开始时的过渡时期,此时速率可以提高或降低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号