当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and biological activity evaluation of dolastatin 10 analogues with N-terminal modifications
Tetrahedron ( IF 2.1 ) Pub Date : 2017-03-26 00:02:31 Xin Wang, Suzhen Dong, Dengke Feng, Yazhou Chen, Mingliang Ma, Wenhao Hu
Tetrahedron ( IF 2.1 ) Pub Date : 2017-03-26 00:02:31 Xin Wang, Suzhen Dong, Dengke Feng, Yazhou Chen, Mingliang Ma, Wenhao Hu
|
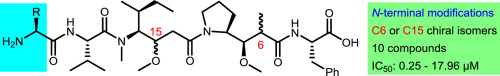
We have described the synthesis of the two complex units (2R,3R,4S)-dolaproine (Dap) and (3R,4S,5S)-dolaisoleuine (Dil) of dolastatin 10 from natural amino acids. The stereoselective syntheses of N-Boc-Dap (4a) and N-Boc-(2S)-iso-Dap (4b) were performed by employing crotylation of N-Boc-l-prolinal as a key step. Barbier-type allylation of N-Boc-l-isoleucinal provided a mild and convenient approach for the synthesis of N-Boc-Dil (5a) and N-Boc-(3S)-iso-Dil (5b). Ten dolastatin 10 analogues have been designed and synthesized with N-terminal modifications based on the known compound monomethylauristatin F (MMAF, 3). In comparison with MMAF (3), four of the compounds showed enhanced potency against HCT 116 human colon cancer cells in vitro.
中文翻译:

具有N末端修饰的dolastatin 10类似物的合成和生物活性评估
我们已经描述了由天然氨基酸合成dolastatin 10的两个复杂单元(2R,3R,4S)-dolaproine(Dap)和(3R,4S,5S)-dolaisoleuine(Dil)的方法。N-Boc-Dap(4a)和N-Boc-(2S)-iso-Dap(4b)的立体选择性合成是通过将N-Boc-1-脯氨酸的crotylation作为关键步骤进行的。N-Boc-1-异亮氨酸的Barbier型烯丙基化为合成N-Boc-Dil(5a)和N-Boc-(3S)-iso-Dil(5b)提供了一种温和而方便的方法。基于已知的化合物单甲基auristatin F(MMAF,3),已经设计并合成了具有N末端修饰的十个dolastatin 10类似物。与MMAF(3)相比,其中四种化合物在体外显示出针对HCT 116人结肠癌细胞的增强效力。
更新日期:2017-03-26
中文翻译:

具有N末端修饰的dolastatin 10类似物的合成和生物活性评估
我们已经描述了由天然氨基酸合成dolastatin 10的两个复杂单元(2R,3R,4S)-dolaproine(Dap)和(3R,4S,5S)-dolaisoleuine(Dil)的方法。N-Boc-Dap(4a)和N-Boc-(2S)-iso-Dap(4b)的立体选择性合成是通过将N-Boc-1-脯氨酸的crotylation作为关键步骤进行的。N-Boc-1-异亮氨酸的Barbier型烯丙基化为合成N-Boc-Dil(5a)和N-Boc-(3S)-iso-Dil(5b)提供了一种温和而方便的方法。基于已知的化合物单甲基auristatin F(MMAF,3),已经设计并合成了具有N末端修饰的十个dolastatin 10类似物。与MMAF(3)相比,其中四种化合物在体外显示出针对HCT 116人结肠癌细胞的增强效力。







































 京公网安备 11010802027423号
京公网安备 11010802027423号