当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical Characterization of AP Lyase and m6A Demethylase Activities of Human AlkB Homologue 1 (ALKBH1)
Biochemistry ( IF 2.9 ) Pub Date : 2017-03-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00060 Tina A. Müller 1 , Michael A. Tobar 1 , Madison N. Perian 2 , Robert P. Hausinger 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2017-03-21 00:00:00 , DOI: 10.1021/acs.biochem.7b00060 Tina A. Müller 1 , Michael A. Tobar 1 , Madison N. Perian 2 , Robert P. Hausinger 1, 3
Affiliation
|
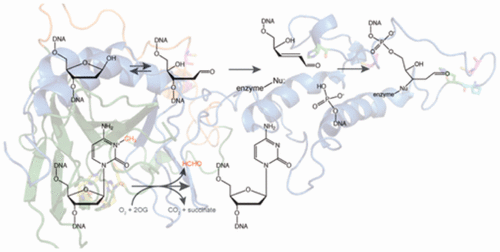
Alkbh1 is one of nine mammalian homologues of Escherichia coli AlkB, a 2-oxoglutarate-dependent dioxygenase that catalyzes direct DNA repair by removing alkyl lesions from DNA. Six distinct enzymatic activities have been reported for Alkbh1, including hydroxylation of variously methylated DNA, mRNA, tRNA, or histone substrates along with the cleavage of DNA at apurinic/apyrimidinic (AP) sites followed by covalent attachment to the 5′-product. The studies described here extend the biochemical characterization of two of these enzymatic activities using human ALKBH1: the AP lyase and 6-methyl adenine DNA demethylase activities. The steady-state and single-turnover kinetic parameters for ALKBH1 cleavage of AP sites in DNA were determined and shown to be comparable to those of other AP lyases. The α,β-unsaturated aldehyde of the 5′-product arising from DNA cleavage reacts predominantly with C129 of ALKBH1, but secondary sites also generate covalent adducts. The 6-methyl adenine demethylase activity was examined with a newly developed assay using a methylation-sensitive restriction endonuclease, and the enzymatic rate was found to be very low. Indeed, the demethylase activity was less than half that of the AP lyase activity when ALKBH1 samples were assayed using identical buffer conditions. The two enzymatic activities were examined using a series of site-directed variant proteins, revealing the presence of distinct but partially overlapping active sites for the two reactions. We postulate that the very low 6-methyl adenine oxygenase activity associated with ALKBH1 is unlikely to represent the major function of the enzyme in the cell, while the cellular role of the lyase activity (including its subsequent covalent attachment to DNA) remains uncertain.
中文翻译:

人类AlkB同源物1(ALKBH1)的AP裂解酶和m 6 A脱甲基酶活性的生化特性
Alkbh1是大肠杆菌的九种哺乳动物同源物中的一种AlkB是一种2-氧戊二酸酯依赖性双加氧酶,可通过从DNA中去除烷基损伤来催化直接DNA修复。已经报道了Alkbh1的六种独特的酶活性,包括各种甲基化的DNA,mRNA,tRNA或组蛋白底物的羟基化,以及在嘌呤/嘧啶(AP)位点的DNA裂解,然后共价附于5'-产物。此处描述的研究使用人ALKBH1扩展了其中两种酶促活性的生物化学特性:AP裂解酶和6-甲基腺嘌呤DNA脱甲基酶活性。确定了ALKBH1裂解DNA中AP位点的稳态和单周转动力学参数,并显示出与其他AP裂解酶相当的动力学参数。DNA裂解产生的5'产物的α,β-不饱和醛主要与ALKBH1的C129反应,但是次要位点也会产生共价加合物。使用甲基化敏感的限制性内切核酸酶,通过新开发的测定法检查了6-甲基腺嘌呤脱甲基酶活性,发现酶促率非常低。实际上,当使用相同的缓冲液条件检测ALKBH1样品时,脱甲基酶活性小于AP裂解酶活性的一半。使用一系列定点的变异蛋白检查了这两种酶的活性,揭示了两个反应存在不同但部分重叠的活性位点。我们推测与ALKBH1相关的6-甲基腺嘌呤氧化酶活性极低,不太可能代表该酶在细胞中的主要功能,
更新日期:2017-03-21
中文翻译:

人类AlkB同源物1(ALKBH1)的AP裂解酶和m 6 A脱甲基酶活性的生化特性
Alkbh1是大肠杆菌的九种哺乳动物同源物中的一种AlkB是一种2-氧戊二酸酯依赖性双加氧酶,可通过从DNA中去除烷基损伤来催化直接DNA修复。已经报道了Alkbh1的六种独特的酶活性,包括各种甲基化的DNA,mRNA,tRNA或组蛋白底物的羟基化,以及在嘌呤/嘧啶(AP)位点的DNA裂解,然后共价附于5'-产物。此处描述的研究使用人ALKBH1扩展了其中两种酶促活性的生物化学特性:AP裂解酶和6-甲基腺嘌呤DNA脱甲基酶活性。确定了ALKBH1裂解DNA中AP位点的稳态和单周转动力学参数,并显示出与其他AP裂解酶相当的动力学参数。DNA裂解产生的5'产物的α,β-不饱和醛主要与ALKBH1的C129反应,但是次要位点也会产生共价加合物。使用甲基化敏感的限制性内切核酸酶,通过新开发的测定法检查了6-甲基腺嘌呤脱甲基酶活性,发现酶促率非常低。实际上,当使用相同的缓冲液条件检测ALKBH1样品时,脱甲基酶活性小于AP裂解酶活性的一半。使用一系列定点的变异蛋白检查了这两种酶的活性,揭示了两个反应存在不同但部分重叠的活性位点。我们推测与ALKBH1相关的6-甲基腺嘌呤氧化酶活性极低,不太可能代表该酶在细胞中的主要功能,





























 京公网安备 11010802027423号
京公网安备 11010802027423号