当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemiluminescence Biosensor Based on 3-D DNA Nanomachine Signal Probe Powered by Protein-Aptamer Binding Complex for Ultrasensitive Mucin 1 Detection
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-03-20 00:00:00 , DOI: 10.1021/acs.analchem.7b00347 Xinya Jiang 1 , Haijun Wang 1 , Huijun Wang 1 , Ying Zhuo 1 , Ruo Yuan 1 , Yaqin Chai 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-03-20 00:00:00 , DOI: 10.1021/acs.analchem.7b00347 Xinya Jiang 1 , Haijun Wang 1 , Huijun Wang 1 , Ying Zhuo 1 , Ruo Yuan 1 , Yaqin Chai 1
Affiliation
|
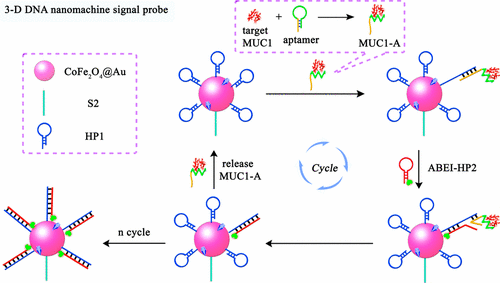
Herein, we fabricated a novel electrochemiluminescence (ECL) biosensor for ultrasensitive detection of mucin 1 (MUC1) based on a three-dimensional (3-D) DNA nanomachine signal probe powered by protein-aptamer binding complex. The assembly of 3-D DNA nanomachine signal probe achieved the cyclic reuse of target protein based on the protein-aptamer binding complex induced catalyzed hairpin assembly (CHA), which overcame the shortcoming of protein conversion with enzyme cleavage or polymerization in the traditional examination of protein. In addition, CoFe2O4, a mimic peroxidase, was used as the nanocarrier of the 3-D DNA nanomachine signal probe to catalyze the decomposition of coreactant H2O2 to generate numerous reactive hydroxyl radical OH• as the efficient accelerator of N-(aminobutyl)-N-(ethylisoluminol) (ABEI) ECL reaction to amplify the luminescence signal. Simultaneously, the assembly of 3-D DNA nanomachine signal probe was executed in solution, which led to abundant luminophore ABEI be immobilized around the CoFe2O4 surface with amplified ECL signal output since the CHA reaction was occurred unencumberedly in all directions under homogeneous environment. The prepared ECL biosensor showed a favorable linear response for MUC1 detection with a relatively low detection limit of 0.62 fg mL–1. With excellent sensitivity, the strategy may provide an efficient method for clinical application, especially in trace protein determination.
中文翻译:

基于蛋白质-适体结合复合物的3-D DNA纳米机信号探针电化学发光生物传感器用于超敏Mucin 1检测。
在本文中,我们基于蛋白质-适体结合复合物驱动的三维(3-D)DNA纳米机器信号探针,制造了一种新型的电化学发光(ECL)生物传感器,用于黏蛋白1(MUC1)的超灵敏检测。3-D DNA纳米机器信号探针的组装基于蛋白质-适体结合复合物诱导的催化发夹组装(CHA),实现了目标蛋白质的循环再利用,克服了传统方法对蛋白质的转化,包括酶裂解或聚合的缺点。蛋白质。此外,模拟过氧化物酶CoFe 2 O 4被用作3-D DNA纳米机器信号探针的纳米载体,以催化共反应剂H 2 O 2的分解。产生大量的反应性羟基自由基•作为N-(氨基丁基)-N-(乙基异鲁米诺)(ABEI)ECL反应的有效促进剂,以放大发光信号。同时,在溶液中进行3-D DNA纳米机信号探针的组装,由于CHA反应在均匀的环境下无方向性地发生,导致大量的发光体ABEI固定在CoFe 2 O 4表面上,并带有放大的ECL信号输出。 。制备的ECL生物传感器对MUC1检测具有良好的线性响应,检测限相对较低,仅为0.62 fg mL –1。该策略具有出色的灵敏度,可以为临床应用提供有效的方法,尤其是在痕量蛋白质测定中。
更新日期:2017-03-20
中文翻译:

基于蛋白质-适体结合复合物的3-D DNA纳米机信号探针电化学发光生物传感器用于超敏Mucin 1检测。
在本文中,我们基于蛋白质-适体结合复合物驱动的三维(3-D)DNA纳米机器信号探针,制造了一种新型的电化学发光(ECL)生物传感器,用于黏蛋白1(MUC1)的超灵敏检测。3-D DNA纳米机器信号探针的组装基于蛋白质-适体结合复合物诱导的催化发夹组装(CHA),实现了目标蛋白质的循环再利用,克服了传统方法对蛋白质的转化,包括酶裂解或聚合的缺点。蛋白质。此外,模拟过氧化物酶CoFe 2 O 4被用作3-D DNA纳米机器信号探针的纳米载体,以催化共反应剂H 2 O 2的分解。产生大量的反应性羟基自由基•作为N-(氨基丁基)-N-(乙基异鲁米诺)(ABEI)ECL反应的有效促进剂,以放大发光信号。同时,在溶液中进行3-D DNA纳米机信号探针的组装,由于CHA反应在均匀的环境下无方向性地发生,导致大量的发光体ABEI固定在CoFe 2 O 4表面上,并带有放大的ECL信号输出。 。制备的ECL生物传感器对MUC1检测具有良好的线性响应,检测限相对较低,仅为0.62 fg mL –1。该策略具有出色的灵敏度,可以为临床应用提供有效的方法,尤其是在痕量蛋白质测定中。































 京公网安备 11010802027423号
京公网安备 11010802027423号