当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amine‐Triggered 6π‐Electrocyclization–Aromatization Cascade of Ynedienamines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-03-20 , DOI: 10.1002/adsc.201601146 Xijian Li 1, 2 , Huidong Yu 3 , Yong Huang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-03-20 , DOI: 10.1002/adsc.201601146 Xijian Li 1, 2 , Huidong Yu 3 , Yong Huang 1
Affiliation
|
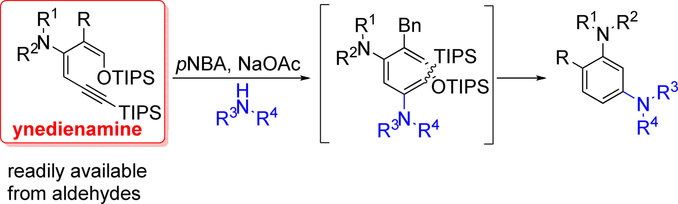
An unprecedented 6π‐electrocyclization–aromatization cascade reaction of ynedienamines is described. The electron‐rich ynedienamine is converted by γ‐protonation to an electrophilic allenyl iminium species which is susceptible to amine addition generating a highly electron‐rich triene intermediate. The 6π‐electrocyclization is accelerated by three electron‐donating substituents in a captodative manner. Subsequent redox‐neutral aromatization allows the direct synthesis of 1,3‐diaminobenzenes from readily available ynedienamines.
中文翻译:

胺引发的6π-电环化-芳基化胺级联反应
炔联胺史无前例的6π-电环化-芳构化级联反应被描述。富电子的炔胺通过γ质子化转化为亲电子的烯基亚胺基亚胺,易于加成胺,生成高度电子富集的三烯中间体。三个给电子的取代基以俘获性方式加速了6π电环化。随后的氧化还原中性芳构化可以从容易获得的炔二胺直接合成1,3-二氨基苯。
更新日期:2017-03-20
中文翻译:

胺引发的6π-电环化-芳基化胺级联反应
炔联胺史无前例的6π-电环化-芳构化级联反应被描述。富电子的炔胺通过γ质子化转化为亲电子的烯基亚胺基亚胺,易于加成胺,生成高度电子富集的三烯中间体。三个给电子的取代基以俘获性方式加速了6π电环化。随后的氧化还原中性芳构化可以从容易获得的炔二胺直接合成1,3-二氨基苯。































 京公网安备 11010802027423号
京公网安备 11010802027423号