当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A positive feedback strategy for enhanced chemotherapy based on ROS-triggered self-accelerating drug release nanosystem
Biomaterials ( IF 12.8 ) Pub Date : 2017-03-25 19:10:45
Jing-Jing Hu, Qi Lei, Meng-Yun Peng, Di-Wei Zheng, Yi-Xuan Chen, Xian-Zheng Zhang
Biomaterials ( IF 12.8 ) Pub Date : 2017-03-25 19:10:45
Jing-Jing Hu, Qi Lei, Meng-Yun Peng, Di-Wei Zheng, Yi-Xuan Chen, Xian-Zheng Zhang
|
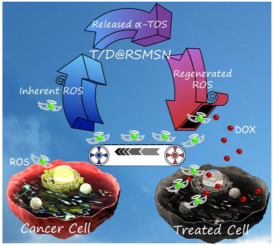
Here, a positive feedback strategy was utilized to amplify the concentration of intracellular reactive oxygen species (ROS) and a ROS-triggered self-accelerating drug release nanosystem (defined as T/D@RSMSNs) was demonstrated for enhanced tumor chemotherapy. The mesoporous silica nanoparticles (MSNs) based nanocarriers were gated by β-cyclodextrin (β-CD) through the ROS-cleavable thioketal (TK) linker to encapsulate the anticancer drug doxorubicin hydrochloride (DOX) and ROS producing agent α-tocopheryl succinate (α-TOS), whose surface was further anchored with adamantane conjugated poly(ethylene glycol) chain (AD-PEG) via host-guest interaction. It was found that in human breast cancer (MCF-7) cells, T/D@RSMSNs could not only release DOX and α-TOS initiatively, but also lead to increased concentration of intracellular ROS, which could be used as new trigger to cut away TK linkage and then in turn facilitate the further release of DOX for enhanced chemotherapy. Both in vitro and in vivo experiments demonstrated that T/D@RSMSNs exhibited more significant antitumor activity in the human breast cancer than the traditional single-DOX loaded ROS-responsive nanocarrier. This novel ROS-triggered self-accelerating drug release nanosystem with remarkably improved therapeutic effects could provide a general strategy to branch out the applications of existing ROS-responsive drug delivery systems (DDSs).
中文翻译:

基于ROS触发的自加速药物释放纳米系统的增强化疗的正反馈策略
在这里,一个积极的反馈策略被用来放大细胞内活性氧(ROS)的浓度,并证明了ROS触发的自加速药物释放纳米系统(定义为T / D @ RSMSNs)可增强肿瘤化学疗法。基于介孔二氧化硅纳米粒子(MSNs)的纳米载体通过ROS可裂解的硫缩酮(TK)接头被β-环糊精(β-CD)门控以封装抗癌药物盐酸阿霉素(DOX)和ROS产生剂α-生育酚琥珀酸酯(α -TOS),其表面通过宿主-客体相互作用进一步锚定于金刚烷共轭聚(乙二醇)链(AD-PEG)。发现在人乳腺癌(MCF-7)细胞中,T / D @ RSMSNs不仅可以主动释放DOX和α-TOS,而且可以导致细胞内ROS浓度增加,可以用作切断传统知识联系的新诱因,进而促进DOX的进一步释放,从而增强化疗效果。体外和体内实验均表明,T / D @ RSMSNs在人乳腺癌中比传统的单一DOX负载的ROS响应纳米载体表现出更显着的抗肿瘤活性。这种新型的由ROS触发的自加速药物释放纳米系统具有显着改善的治疗效果,可以为推广现有ROS响应药物递送系统(DDS)的应用提供一种通用策略。体外和体内实验均表明,T / D @ RSMSNs在人乳腺癌中比传统的单一DOX负载的ROS响应纳米载体表现出更显着的抗肿瘤活性。这种新型的由ROS触发的自加速药物释放纳米系统具有显着改善的治疗效果,可以为推广现有ROS响应药物递送系统(DDS)的应用提供一种通用策略。体外和体内实验均表明,T / D @ RSMSNs在人乳腺癌中比传统的单一DOX负载的ROS响应纳米载体表现出更显着的抗肿瘤活性。这种新型的由ROS触发的自加速药物释放纳米系统具有显着改善的治疗效果,可以为推广现有ROS响应药物递送系统(DDS)的应用提供一种通用策略。
更新日期:2017-03-26
中文翻译:

基于ROS触发的自加速药物释放纳米系统的增强化疗的正反馈策略
在这里,一个积极的反馈策略被用来放大细胞内活性氧(ROS)的浓度,并证明了ROS触发的自加速药物释放纳米系统(定义为T / D @ RSMSNs)可增强肿瘤化学疗法。基于介孔二氧化硅纳米粒子(MSNs)的纳米载体通过ROS可裂解的硫缩酮(TK)接头被β-环糊精(β-CD)门控以封装抗癌药物盐酸阿霉素(DOX)和ROS产生剂α-生育酚琥珀酸酯(α -TOS),其表面通过宿主-客体相互作用进一步锚定于金刚烷共轭聚(乙二醇)链(AD-PEG)。发现在人乳腺癌(MCF-7)细胞中,T / D @ RSMSNs不仅可以主动释放DOX和α-TOS,而且可以导致细胞内ROS浓度增加,可以用作切断传统知识联系的新诱因,进而促进DOX的进一步释放,从而增强化疗效果。体外和体内实验均表明,T / D @ RSMSNs在人乳腺癌中比传统的单一DOX负载的ROS响应纳米载体表现出更显着的抗肿瘤活性。这种新型的由ROS触发的自加速药物释放纳米系统具有显着改善的治疗效果,可以为推广现有ROS响应药物递送系统(DDS)的应用提供一种通用策略。体外和体内实验均表明,T / D @ RSMSNs在人乳腺癌中比传统的单一DOX负载的ROS响应纳米载体表现出更显着的抗肿瘤活性。这种新型的由ROS触发的自加速药物释放纳米系统具有显着改善的治疗效果,可以为推广现有ROS响应药物递送系统(DDS)的应用提供一种通用策略。体外和体内实验均表明,T / D @ RSMSNs在人乳腺癌中比传统的单一DOX负载的ROS响应纳米载体表现出更显着的抗肿瘤活性。这种新型的由ROS触发的自加速药物释放纳米系统具有显着改善的治疗效果,可以为推广现有ROS响应药物递送系统(DDS)的应用提供一种通用策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号