当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism-Based Inhibition of the Mycobacterium tuberculosis Branched-Chain Aminotransferase by d- and l-Cycloserine
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-03-16 00:00:00 , DOI: 10.1021/acschembio.7b00142
Tathyana Mar Amorim Franco 1 , Lorenza Favrot 1 , Olivia Vergnolle 1 , John S. Blanchard 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-03-16 00:00:00 , DOI: 10.1021/acschembio.7b00142
Tathyana Mar Amorim Franco 1 , Lorenza Favrot 1 , Olivia Vergnolle 1 , John S. Blanchard 1
Affiliation
|
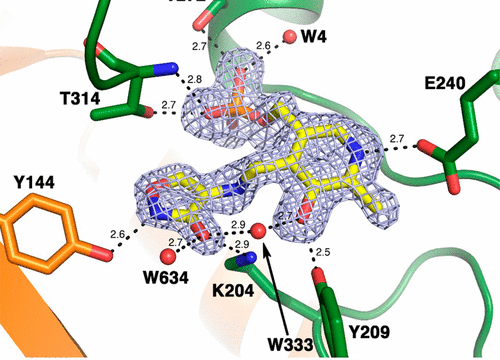
The branched-chain aminotransferase is a pyridoxal 5′-phosphate (PLP)-dependent enzyme responsible for the final step in the biosynthesis of all three branched-chain amino acids, l-leucine, l-isoleucine, and l-valine, in bacteria. We have investigated the mechanism of inactivation of the branched-chain aminotransferase from Mycobacterium tuberculosis (MtIlvE) by d- and l-cycloserine. d-Cycloserine is currently used only in the treatment of multidrug–drug-resistant tuberculosis. Our results show a time- and concentration-dependent inactivation of MtIlvE by both isomers, with l-cycloserine being a 40-fold better inhibitor of the enzyme. Minimum inhibitory concentration (MIC) studies revealed that l-cycloserine is a 10-fold better inhibitor of Mycobacterium tuberculosis growth than d-cycloserine. In addition, we have crystallized the MtIlvE-d-cycloserine inhibited enzyme, determining the structure to 1.7 Å. The structure of the covalent d-cycloserine-PMP adduct bound to MtIlvE reveals that the d-cycloserine ring is planar and aromatic, as previously observed for other enzyme systems. Mass spectrometry reveals that both the d-cycloserine- and l-cycloserine-PMP complexes have the same mass, and are likely to be the same aromatized, isoxazole product. However, the kinetics of formation of the MtIlvE d-cycloserine-PMP and MtIlvE l-cycloserine-PMP adducts are quite different. While the kinetics of the formation of the MtIlvE d-cycloserine-PMP complex can be fit to a single exponential, the formation of the MtIlvE l-cycloserine-PMP complex occurs in two steps. We propose a chemical mechanism for the inactivation of d- and l-cycloserine which suggests a stereochemically determined structural role for the differing kinetics of inactivation. These results demonstrate that the mechanism of action of d-cycloserine’s activity against M. tuberculosis may be more complicated than previously thought and that d-cycloserine may compromise the in vivo activity of multiple PLP-dependent enzymes, including MtIlvE.
中文翻译:

d-和l-环丝氨酸对结核分枝杆菌分支链氨基转移酶的机制抑制
支链氨基转移酶是一种吡咯醛5'-磷酸(PLP)依赖性酶,负责细菌中所有三个支链氨基酸(1-亮氨酸,1-异亮氨酸和1-缬氨酸)的生物合成的最终步骤。。我们研究了d-和l-环丝氨酸使结核分枝杆菌(Mt IlvE)的支链氨基转移酶失活的机制。d-环丝氨酸目前仅用于治疗耐多药结核病。我们的结果显示的时间和浓度依赖性失活山IlvE由两种异构体,与升-环丝氨酸是该酶的40倍更好的抑制剂。最小抑菌浓度(MIC)研究表明,1-环丝氨酸是结核分枝杆菌生长的10倍,比d-环丝氨酸好10倍。另外,我们已经结晶了Mt IlvE- d-环丝氨酸抑制的酶,将结构确定为1.7。结合到Mt IlvE上的共价d-环丝氨酸-PMP加合物的结构揭示了d-环丝氨酸环是平面的并且是芳香族的,正如先前在其他酶系统中观察到的那样。质谱显示d-环丝氨酸和l-环丝氨酸-PMP复合物具有相同的质量,并且可能是相同的芳香化异恶唑产品。然而,Mt IlvE d-环丝氨酸-PMP和Mt IlvE 1-环丝氨酸-PMP加合物形成的动力学是完全不同的。而形成的动力学山IlvE ð -cycloserine-PMP络合物可以是适合于单指数,所述的形成山IlvE升-cycloserine-PMP复合发生在两个步骤。我们提出了使d-和l失活的化学机制-环丝氨酸暗示了不同灭活动力学的立体化学确定的结构作用。这些结果表明,d-环丝氨酸对结核分枝杆菌的作用机理可能比以前认为的更为复杂,并且d-环丝氨酸可能损害包括Mt IlvE在内的多种PLP依赖性酶的体内活性。
更新日期:2017-03-16
中文翻译:

d-和l-环丝氨酸对结核分枝杆菌分支链氨基转移酶的机制抑制
支链氨基转移酶是一种吡咯醛5'-磷酸(PLP)依赖性酶,负责细菌中所有三个支链氨基酸(1-亮氨酸,1-异亮氨酸和1-缬氨酸)的生物合成的最终步骤。。我们研究了d-和l-环丝氨酸使结核分枝杆菌(Mt IlvE)的支链氨基转移酶失活的机制。d-环丝氨酸目前仅用于治疗耐多药结核病。我们的结果显示的时间和浓度依赖性失活山IlvE由两种异构体,与升-环丝氨酸是该酶的40倍更好的抑制剂。最小抑菌浓度(MIC)研究表明,1-环丝氨酸是结核分枝杆菌生长的10倍,比d-环丝氨酸好10倍。另外,我们已经结晶了Mt IlvE- d-环丝氨酸抑制的酶,将结构确定为1.7。结合到Mt IlvE上的共价d-环丝氨酸-PMP加合物的结构揭示了d-环丝氨酸环是平面的并且是芳香族的,正如先前在其他酶系统中观察到的那样。质谱显示d-环丝氨酸和l-环丝氨酸-PMP复合物具有相同的质量,并且可能是相同的芳香化异恶唑产品。然而,Mt IlvE d-环丝氨酸-PMP和Mt IlvE 1-环丝氨酸-PMP加合物形成的动力学是完全不同的。而形成的动力学山IlvE ð -cycloserine-PMP络合物可以是适合于单指数,所述的形成山IlvE升-cycloserine-PMP复合发生在两个步骤。我们提出了使d-和l失活的化学机制-环丝氨酸暗示了不同灭活动力学的立体化学确定的结构作用。这些结果表明,d-环丝氨酸对结核分枝杆菌的作用机理可能比以前认为的更为复杂,并且d-环丝氨酸可能损害包括Mt IlvE在内的多种PLP依赖性酶的体内活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号