当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Reduction of Ferric Porphyrins by Sulfide: Identification of a Low Spin FeIII–SH Intermediate
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-03-15 00:00:00 , DOI: 10.1021/acs.inorgchem.6b02878 Kaustuv Mittra 1 , Asmita Singha 1 , Abhishek Dey 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-03-15 00:00:00 , DOI: 10.1021/acs.inorgchem.6b02878 Kaustuv Mittra 1 , Asmita Singha 1 , Abhishek Dey 1
Affiliation
|
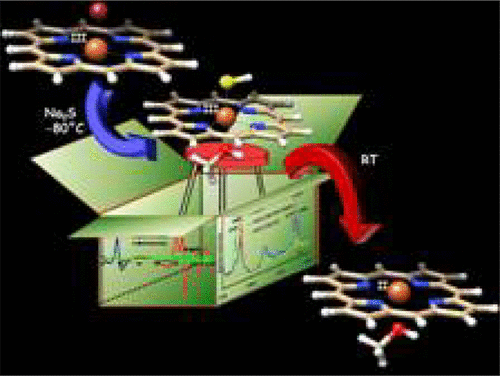
The reaction of FeIII porphyrin complexes bearing distal hydrogen bonding residues with sulfide/hydrosulfide is kinetically monitored to reveal the presence of an intermediate and a kH/kD of 3.0. This intermediate is trapped at low temperatures and investigated with resonance Raman and electron paramagnetic resonance spectroscopy. The results, corroborated by density functional theory calculations, indicate that this species is a six-coordinate low spin hydrosulfide bound ferric porphyrin. The homolytic cleavage of the FeIII–SH bond resulting in the formation of a ferrous porphyrin and hydrosulfide radical (trapped with 5,5-dimethyl-1-pyrrilone-N-oxide) is found to be the overall rate-determining step of the reaction.
中文翻译:

硫化物还原铁卟啉的机理:低自旋Fe III -SH中间体的鉴定
动力学监测带有远端氢键残基的Fe III卟啉配合物与硫化物/氢硫化物的反应,以揭示中间体的存在和k H / k D为3.0。该中间体在低温下被捕获,并通过共振拉曼光谱和电子顺磁共振光谱进行研究。由密度泛函理论计算所证实的结果表明,该物种是六配位的低自旋氢硫化物结合的三价铁卟啉。Fe III的均相裂解发现导致金属亚铁卟啉和氢硫化物自由基(被5,5-二甲基-1-吡咯烷酮-N-氧化物捕获)的-SH键是反应的总体速率决定步骤。
更新日期:2017-03-15
中文翻译:

硫化物还原铁卟啉的机理:低自旋Fe III -SH中间体的鉴定
动力学监测带有远端氢键残基的Fe III卟啉配合物与硫化物/氢硫化物的反应,以揭示中间体的存在和k H / k D为3.0。该中间体在低温下被捕获,并通过共振拉曼光谱和电子顺磁共振光谱进行研究。由密度泛函理论计算所证实的结果表明,该物种是六配位的低自旋氢硫化物结合的三价铁卟啉。Fe III的均相裂解发现导致金属亚铁卟啉和氢硫化物自由基(被5,5-二甲基-1-吡咯烷酮-N-氧化物捕获)的-SH键是反应的总体速率决定步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号