当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Cation−π Interaction Enables a Halo-Tag Fluorogenic Probe for Fast No-Wash Live Cell Imaging and Gel-Free Protein Quantification
Biochemistry ( IF 2.9 ) Pub Date : 2017-03-13 00:00:00 , DOI: 10.1021/acs.biochem.7b00056
Yu Liu , Kun Miao , Noah P. Dunham , Hongbin Liu 1 , Matthew Fares , Amie K. Boal , Xiaosong Li 1 , Xin Zhang
Biochemistry ( IF 2.9 ) Pub Date : 2017-03-13 00:00:00 , DOI: 10.1021/acs.biochem.7b00056
Yu Liu , Kun Miao , Noah P. Dunham , Hongbin Liu 1 , Matthew Fares , Amie K. Boal , Xiaosong Li 1 , Xin Zhang
Affiliation
|
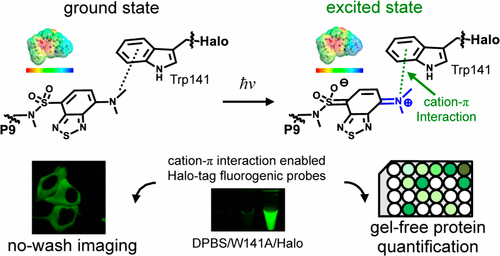
The design of fluorogenic probes for a Halo tag is highly desirable but challenging. Previous work achieved this goal by controlling the chemical switch of spirolactones upon the covalent conjugation between the Halo tag and probes or by incorporating a “channel dye” into the substrate binding tunnel of the Halo tag. In this work, we have developed a novel class of Halo-tag fluorogenic probes that are derived from solvatochromic fluorophores. The optimal probe, harboring a benzothiadiazole scaffold, exhibits a 1000-fold fluorescence enhancement upon reaction with the Halo tag. Structural, computational, and biochemical studies reveal that the benzene ring of a tryptophan residue engages in a cation−π interaction with the dimethylamino electron-donating group of the benzothiadiazole fluorophore in its excited state. We further demonstrate using noncanonical fluorinated tryptophan that the cation−π interaction directly contributes to the fluorogenicity of the benzothiadiazole fluorophore. Mechanistically, this interaction could contribute to the fluorogenicity by promoting the excited-state charge separation and inhibiting the twisting motion of the dimethylamino group, both leading to an enhanced fluorogenicity. Finally, we demonstrate the utility of the probe in no-wash direct imaging of Halo-tagged proteins in live cells. In addition, the fluorogenic nature of the probe enables a gel-free quantification of fusion proteins expressed in mammalian cells, an application that was not possible with previously nonfluorogenic Halo-tag probes. The unique mechanism revealed by this work suggests that incorporation of an excited-state cation−π interaction could be a feasible strategy for enhancing the optical performance of fluorophores and fluorogenic sensors.
中文翻译:

阳离子-π相互作用使Halo-Tag荧光探针实现快速无洗活细胞成像和无凝胶蛋白定量
非常需要用于Halo标签的荧光探针的设计,但是具有挑战性。先前的工作通过控制Halo标签与探针之间的共价缀合来控制螺内酯的化学转换,或通过将“通道染料”掺入Halo标签的底物结合通道中来实现此目标。在这项工作中,我们已经开发出一类新的Halo-tag荧光探针,这些探针衍生自溶剂变色荧光团。带有苯并噻二唑支架的最佳探针在与Halo标签反应后显示出1000倍的荧光增强。结构,计算和生化研究表明,色氨酸残基的苯环与处于激发态的苯并噻二唑荧光团的二甲基氨基给电子基团发生阳离子-π相互作用。我们进一步证明了使用非规范的氟化色氨酸,阳离子-π相互作用直接促进了苯并噻二唑荧光团的荧光性。从机理上讲,这种相互作用可通过促进激发态电荷分离和抑制二甲基氨基的扭转运动来促进荧光性,两者均导致增强的荧光性。最后,我们证明了该探针在活细胞中Halo标记蛋白的免洗直接成像中的实用性。此外,该探针的荧光性质使得能够对哺乳动物细胞中表达的融合蛋白进行无凝胶定量,而以前的非荧光Halo-tag探针无法实现这种应用。
更新日期:2017-03-13
中文翻译:

阳离子-π相互作用使Halo-Tag荧光探针实现快速无洗活细胞成像和无凝胶蛋白定量
非常需要用于Halo标签的荧光探针的设计,但是具有挑战性。先前的工作通过控制Halo标签与探针之间的共价缀合来控制螺内酯的化学转换,或通过将“通道染料”掺入Halo标签的底物结合通道中来实现此目标。在这项工作中,我们已经开发出一类新的Halo-tag荧光探针,这些探针衍生自溶剂变色荧光团。带有苯并噻二唑支架的最佳探针在与Halo标签反应后显示出1000倍的荧光增强。结构,计算和生化研究表明,色氨酸残基的苯环与处于激发态的苯并噻二唑荧光团的二甲基氨基给电子基团发生阳离子-π相互作用。我们进一步证明了使用非规范的氟化色氨酸,阳离子-π相互作用直接促进了苯并噻二唑荧光团的荧光性。从机理上讲,这种相互作用可通过促进激发态电荷分离和抑制二甲基氨基的扭转运动来促进荧光性,两者均导致增强的荧光性。最后,我们证明了该探针在活细胞中Halo标记蛋白的免洗直接成像中的实用性。此外,该探针的荧光性质使得能够对哺乳动物细胞中表达的融合蛋白进行无凝胶定量,而以前的非荧光Halo-tag探针无法实现这种应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号