当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid-Phase H-Transfer from 2-Propanol to Phenol on Raney Ni: Surface Processes and Inhibition
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-03-03 00:00:00 , DOI: 10.1021/acscatal.6b03201
Marco Kennema 1 , Ilton Barros Daltro de Castro 1 , Fabian Meemken 2 , Roberto Rinaldi 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-03-03 00:00:00 , DOI: 10.1021/acscatal.6b03201
Marco Kennema 1 , Ilton Barros Daltro de Castro 1 , Fabian Meemken 2 , Roberto Rinaldi 3
Affiliation
|
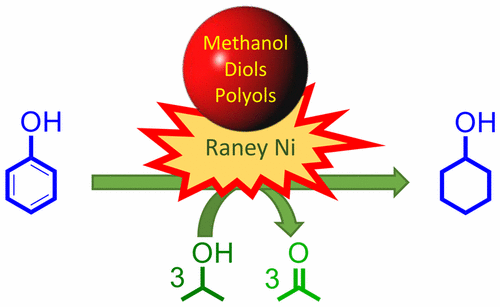
Raney Ni is perhaps the most widely used catalyst for the transformation of biogenic molecules in industrial practice (e.g., as in the production of sugar alcohols and hardening of vegetable oils). Currently, Raney Ni has found another key application; the catalytic upstream biorefining (CUB) of lignocellulose in which the soluble products released from the lignocellulosic matrix undergo reductive processes, rendering depolymerized lignin oils in addition to high-quality holocellulosic pulps. Despite the industrial importance of Raney Ni, its surface chemistry is poorly understood. In this study, using the H-transfer reaction between 2-propanol (2-PrOH) and phenol as a model reaction, we studied the influence of various alcohols on the catalytic performance of Raney Ni. For the H-transfer hydrogenation of phenol to cyclohexanol, the inhibition of the catalyst increases in the order of secondary alcohols < primary alcohols < polyols at 130 °C. To better understand the observed inhibition, we also studied the molecular interactions of the various alcohols at the catalytic solid–liquid interface using in situ attenuated total reflection infrared (ATR-IR) spectroscopy. The in situ spectroscopic data revealed that 2-PrOH adsorbs on at least two chemically different sites on the surface of Raney Ni. One of these two adsorption sites was attributed to the Ni site responsible for the saturation of the phenolic ring. The ATR-IR spectroscopic data also shows that the adsorption of phenol involves its hydroxyl group. Notably, the phenolic ring was found to be tilted with respect to the surface. Competitive adsorption of various other alcohols was also investigated at the catalytic solid–liquid interface. The presence of methanol inhibited the adsorption of 2-PrOH to a significantly greater degree than phenol. Therefore, it is proposed that hydrogen transfer hydrogenation of the phenolic ring is inhibited in the presence of additional alcohols mainly due to the competitive adsorption with 2-PrOH. Several polyols were found to interact through a bidentate interaction with the catalyst surface, which explains their stronger inhibition compared to primary alcohols. In a broader context, this study proposes the effect of hemicellulose sugars and sugar alcohols, formed in the CUB process, upon the product selectivity of CUB catalyzed by Raney Ni and using 2-PrOH as an H-donor.
中文翻译:

在阮内镍上从2-丙醇向苯酚的液相H转移:表面过程和抑制
Raney Ni可能是工业实践中用于转化生物分子的最广泛使用的催化剂(例如,如糖醇的生产和植物油的硬化)。目前,阮内妮(Raney Ni)找到了另一个关键应用程序;木质纤维素的催化上游生物精制(CUB),其中从木质纤维素基质释放的可溶性产物经过还原过程,除提供优质的全纤维素纸浆外,还提供了解聚的木质素油。尽管阮内镍在工业上很重要,但对其表面化学的了解却很少。在这项研究中,以2-丙醇(2-PrOH)与苯酚之间的H转移反应为模型反应,我们研究了各种醇类对阮内镍催化性能的影响。对于苯酚的H-转移加氢为环己醇,在130°C下,催化剂的抑制作用按仲醇<伯醇<多元醇的顺序增加。为了更好地理解所观察到的抑制作用,我们还使用原位衰减全反射红外(ATR-IR)光谱研究了固醇催化界面处各种醇类的分子相互作用。原位光谱数据表明2-PrOH吸附在阮内镍表面上至少两个化学不同的位点上。这两个吸附位点之一归因于负责酚环饱和的Ni位点。ATR-IR光谱数据还表明苯酚的吸附涉及其羟基。特别地,发现酚环相对于表面倾斜。还研究了催化固液界面上其他各种醇的竞争性吸附。甲醇的存在抑制了2-PrOH的吸附,其吸附程度明显高于苯酚。因此,提出主要由于与2-PrOH的竞争性吸附而在另外的醇的存在下抑制了酚环的氢转移氢化。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。甲醇的存在抑制了2-PrOH的吸附,其吸附程度明显高于苯酚。因此,提出主要由于与2-PrOH的竞争性吸附而在另外的醇的存在下抑制了酚环的氢转移氢化。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。甲醇的存在抑制了2-PrOH的吸附,其吸附程度明显高于苯酚。因此,提出主要由于与2-PrOH的竞争性吸附而在另外的醇的存在下抑制了酚环的氢转移氢化。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。有人提出,在其他醇的存在下,酚环的氢转移加氢被抑制,这主要是由于与2-PrOH的竞争性吸附。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。有人提出,在其他醇的存在下,酚环的氢转移加氢被抑制,这主要是由于与2-PrOH的竞争性吸附。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。
更新日期:2017-03-03
中文翻译:

在阮内镍上从2-丙醇向苯酚的液相H转移:表面过程和抑制
Raney Ni可能是工业实践中用于转化生物分子的最广泛使用的催化剂(例如,如糖醇的生产和植物油的硬化)。目前,阮内妮(Raney Ni)找到了另一个关键应用程序;木质纤维素的催化上游生物精制(CUB),其中从木质纤维素基质释放的可溶性产物经过还原过程,除提供优质的全纤维素纸浆外,还提供了解聚的木质素油。尽管阮内镍在工业上很重要,但对其表面化学的了解却很少。在这项研究中,以2-丙醇(2-PrOH)与苯酚之间的H转移反应为模型反应,我们研究了各种醇类对阮内镍催化性能的影响。对于苯酚的H-转移加氢为环己醇,在130°C下,催化剂的抑制作用按仲醇<伯醇<多元醇的顺序增加。为了更好地理解所观察到的抑制作用,我们还使用原位衰减全反射红外(ATR-IR)光谱研究了固醇催化界面处各种醇类的分子相互作用。原位光谱数据表明2-PrOH吸附在阮内镍表面上至少两个化学不同的位点上。这两个吸附位点之一归因于负责酚环饱和的Ni位点。ATR-IR光谱数据还表明苯酚的吸附涉及其羟基。特别地,发现酚环相对于表面倾斜。还研究了催化固液界面上其他各种醇的竞争性吸附。甲醇的存在抑制了2-PrOH的吸附,其吸附程度明显高于苯酚。因此,提出主要由于与2-PrOH的竞争性吸附而在另外的醇的存在下抑制了酚环的氢转移氢化。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。甲醇的存在抑制了2-PrOH的吸附,其吸附程度明显高于苯酚。因此,提出主要由于与2-PrOH的竞争性吸附而在另外的醇的存在下抑制了酚环的氢转移氢化。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。甲醇的存在抑制了2-PrOH的吸附,其吸附程度明显高于苯酚。因此,提出主要由于与2-PrOH的竞争性吸附而在另外的醇的存在下抑制了酚环的氢转移氢化。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。有人提出,在其他醇的存在下,酚环的氢转移加氢被抑制,这主要是由于与2-PrOH的竞争性吸附。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。有人提出,在其他醇的存在下,酚环的氢转移加氢被抑制,这主要是由于与2-PrOH的竞争性吸附。发现几种多元醇通过双齿相互作用与催化剂表面相互作用,这解释了它们与伯醇相比具有更强的抑制作用。在更广泛的背景下,这项研究提出了在CUB过程中形成的半纤维素糖和糖醇对阮内妮(Raney Ni)催化并使用2-PrOH作为氢供体的CUB产物选择性的影响。







































 京公网安备 11010802027423号
京公网安备 11010802027423号