Nature Chemical Biology ( IF 12.9 ) Pub Date : 2017-03-06 , DOI: 10.1038/nchembio.2333 Yijin Liu 1 , Timothy J Wilson 1 , David M J Lilley 1
|
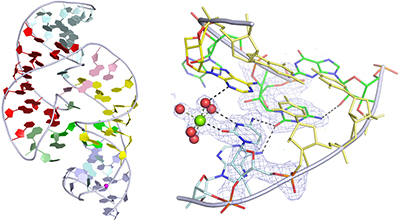
The TS ribozyme (originally called “twister sister”) is a catalytic RNA. We present a crystal structure of the ribozyme in a pre-reactive conformation. Two co-axial helical stacks are organized by a three-way junction and two tertiary contacts. Five divalent metal ions are directly coordinated to RNA ligands, making important contributions to the RNA architecture. The scissile phosphate lies in a quasihelical loop region that is organized by a network of hydrogen bonding. A divalent metal ion is directly bound to the nucleobase 5′ to the scissile phosphate, with an inner-sphere water molecule positioned to interact with the O2′ nucleophile. The rate of ribozyme cleavage correlated in a log-linear manner with divalent metal ion pKa, consistent with proton transfer in the transition state, and we propose that the bound metal ion is a likely general base for the cleavage reaction. Our data indicate that the TS ribozyme functions predominantly as a metalloenzyme.
中文翻译:

采用催化金属离子的溶核核酶的结构
TS 核酶(最初称为“twisterister”)是一种催化 RNA。我们展示了处于预反应构象的核酶的晶体结构。两个同轴螺旋叠层由三路连接点和两个三级触点组成。五种二价金属离子直接与 RNA 配体配位,对 RNA 结构做出了重要贡献。可裂解的磷酸盐位于由氢键网络组织的准螺旋环区域中。二价金属离子直接与可裂变磷酸盐的核碱基 5' 结合,内层水分子的位置与 O2' 亲核试剂相互作用。核酶裂解速率与二价金属离子pKa以对数线性方式相关,与过渡态的质子转移一致,我们认为结合的金属离子可能是裂解反应的通用基础。我们的数据表明 TS 核酶主要作为金属酶发挥作用。













































 京公网安备 11010802027423号
京公网安备 11010802027423号