当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preventing Alkyne–Alkyne (i.e., Glaser) Coupling Associated with the ATRP Synthesis of Alkyne-Functional Polymers/Macromonomers and for Alkynes under Click (i.e., CuAAC) Reaction Conditions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-06 , DOI: 10.1021/jacs.6b12525 Porakrit Leophairatana 1 , Sanjoy Samanta 1 , Chathuranga C. De Silva 1 , Jeffrey T. Koberstein 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-03-06 , DOI: 10.1021/jacs.6b12525 Porakrit Leophairatana 1 , Sanjoy Samanta 1 , Chathuranga C. De Silva 1 , Jeffrey T. Koberstein 1
Affiliation
|
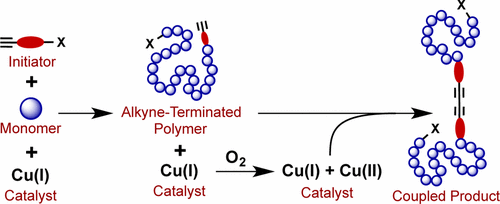
Alkyne-functional polymers synthesized by ATRP exhibit bimodal molecular weight distributions indicating the occurrence of some undesirable side reaction. By modeling the molecular weight distributions obtained under various reaction conditions, we show that the side reaction is alkyne-alkyne (i.e., Glaser) coupling. Glaser coupling accounts for as much as 20% of the polymer produced, significantly compromising the polymer functionality and undermining the success of subsequent click reactions in which they are used. Glaser coupling does not occur during ATRP but during postpolymerization workup upon first exposure to air. Two strategies are reported that effectively eliminate these coupling reactions without the need for a protecting group for the alkyne-functional initiator: (1) maintaining low temperature post-ATRP upon exposure to air followed by immediate removal of copper catalyst; (2) adding excess reducing agents post-ATRP which prevent the oxidation of Cu(I) catalyst required by the Glaser coupling mechanism. Post-ATRP Glaser coupling was also influenced by the ATRP synthesis ligand used. The order of ligand activity for catalyzing Glaser coupling was: linear bidentate > tridentate > tetradentate. We find that Glaser coupling is not problematic in ARGET-ATRP of alkyne-terminated polymers because a reducing agent is present during polymerization, however the molecular weight distribution is broadened compared to ATRP due to the presence of oxygen. Glaser coupling can also occur for alkynes held under CuAAC reaction conditions but again can be eliminated by adding appropriate reducing agents.
中文翻译:

防止与炔功能聚合物/大分子单体的 ATRP 合成相关的炔-炔(即格拉泽)偶联以及在点击(即 CuAAC)反应条件下的炔
由 ATRP 合成的炔官能聚合物表现出双峰分子量分布,表明发生了一些不希望有的副反应。通过模拟在各种反应条件下获得的分子量分布,我们表明副反应是炔-炔(即 Glaser)偶联。Glaser 偶联占所生产聚合物的 20%,显着损害了聚合物的功能,并破坏了随后使用它们的点击反应的成功。格拉泽偶联不会在 ATRP 期间发生,而是在首次暴露于空气后的聚合后处理期间发生。据报道,两种策略可有效消除这些偶联反应,而无需炔烃官能引发剂的保护基团:(1) 暴露于空气后,在 ATRP 后保持低温,然后立即去除铜催化剂;(2) 在 ATRP 后添加过量的还原剂,以防止 Glaser 耦合机制所需的 Cu(I) 催化剂的氧化。ATRP 后 Glaser 偶联也受所用 ATRP 合成配体的影响。催化Glaser偶联的配体活性顺序为:线性二齿>三齿>四齿。我们发现在炔封端聚合物的 ARGET-ATRP 中 Glaser 偶联没有问题,因为在聚合过程中存在还原剂,但是由于存在氧,与 ATRP 相比分子量分布变宽。在 CuAAC 反应条件下保持的炔烃也可能发生 Glaser 偶联,但也可以通过添加适当的还原剂来消除。
更新日期:2017-03-06
中文翻译:

防止与炔功能聚合物/大分子单体的 ATRP 合成相关的炔-炔(即格拉泽)偶联以及在点击(即 CuAAC)反应条件下的炔
由 ATRP 合成的炔官能聚合物表现出双峰分子量分布,表明发生了一些不希望有的副反应。通过模拟在各种反应条件下获得的分子量分布,我们表明副反应是炔-炔(即 Glaser)偶联。Glaser 偶联占所生产聚合物的 20%,显着损害了聚合物的功能,并破坏了随后使用它们的点击反应的成功。格拉泽偶联不会在 ATRP 期间发生,而是在首次暴露于空气后的聚合后处理期间发生。据报道,两种策略可有效消除这些偶联反应,而无需炔烃官能引发剂的保护基团:(1) 暴露于空气后,在 ATRP 后保持低温,然后立即去除铜催化剂;(2) 在 ATRP 后添加过量的还原剂,以防止 Glaser 耦合机制所需的 Cu(I) 催化剂的氧化。ATRP 后 Glaser 偶联也受所用 ATRP 合成配体的影响。催化Glaser偶联的配体活性顺序为:线性二齿>三齿>四齿。我们发现在炔封端聚合物的 ARGET-ATRP 中 Glaser 偶联没有问题,因为在聚合过程中存在还原剂,但是由于存在氧,与 ATRP 相比分子量分布变宽。在 CuAAC 反应条件下保持的炔烃也可能发生 Glaser 偶联,但也可以通过添加适当的还原剂来消除。















































 京公网安备 11010802027423号
京公网安备 11010802027423号