当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of N-benzyl-des-D-ring lamellarin K via an acyl-Claisen/Paal-Knorr approach
Tetrahedron ( IF 2.1 ) Pub Date : 2017-03-21 12:30:51 Nora Dittrich, Lisa I. Pilkington, Euphemia Leung, David Barker
Tetrahedron ( IF 2.1 ) Pub Date : 2017-03-21 12:30:51 Nora Dittrich, Lisa I. Pilkington, Euphemia Leung, David Barker
|
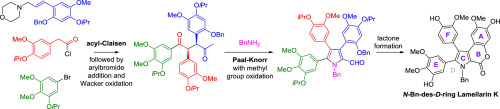
Lamellarin K is a complex pyrrole natural product and member of the lamellarin family – a group of natural products known for their potent biological activities, such as, antiproliferative activity and inhibition of P-gp mediated drug efflux pumps. We herein describe the synthesis of the N-benzyl-des-D ring analogue of lamellarin K using a route that centres on an acyl-Claisen reaction to eventually prepare a highly-functionalised 1-aryl-4-methyl-1,4-diketone. Paal-Knorr pyrrole formation using this diketone undergoes auto-oxidation to give a fully-substituted 5-formyl pyrrole which was converted into the natural lactone B ring. Antiproliferative testing of the N-benzyl-des-D ring analogue gave an IC50 of 2.63 μM against the MDA-MB-231 breast cancer cell line.
中文翻译:

通过酰基克莱森/帕尔-克诺尔方法合成N-苄基-des-D-环薄片
Lamellarin K是复杂的吡咯天然产物,是lamellarin家族的成员-lamellarin家族是一组天然产物,以其强大的生物活性(例如抗增殖活性和对P-gp介导的药物外排泵的抑制作用)而闻名。我们在此描述了lamellarin K的N-苄基-des-D环类似物的合成,其使用以酰基-克莱森反应为中心以最终制备高度官能化的1-芳基-4-甲基-1,4-二酮的途径。使用该二酮形成的Paal-Knorr吡咯经过自动氧化,得到完全取代的5-甲酰基吡咯,其被转化为天然内酯B环。N-苄基-des-D环类似物的抗增殖测试显示,针对MDA-MB-231乳腺癌细胞系的IC 50为2.63μM。
更新日期:2017-03-22
中文翻译:

通过酰基克莱森/帕尔-克诺尔方法合成N-苄基-des-D-环薄片
Lamellarin K是复杂的吡咯天然产物,是lamellarin家族的成员-lamellarin家族是一组天然产物,以其强大的生物活性(例如抗增殖活性和对P-gp介导的药物外排泵的抑制作用)而闻名。我们在此描述了lamellarin K的N-苄基-des-D环类似物的合成,其使用以酰基-克莱森反应为中心以最终制备高度官能化的1-芳基-4-甲基-1,4-二酮的途径。使用该二酮形成的Paal-Knorr吡咯经过自动氧化,得到完全取代的5-甲酰基吡咯,其被转化为天然内酯B环。N-苄基-des-D环类似物的抗增殖测试显示,针对MDA-MB-231乳腺癌细胞系的IC 50为2.63μM。






























 京公网安备 11010802027423号
京公网安备 11010802027423号