当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Some Quinoxalin-6-yl Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-07-06 00:00:00 , DOI: 10.1021/acs.jpcc.5b03285 Lukman O. Olasunkanmi 1, 2, 3 , Ime B. Obot 4 , Mwadham M. Kabanda 1, 2 , Eno E. Ebenso 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-07-06 00:00:00 , DOI: 10.1021/acs.jpcc.5b03285 Lukman O. Olasunkanmi 1, 2, 3 , Ime B. Obot 4 , Mwadham M. Kabanda 1, 2 , Eno E. Ebenso 1, 2
Affiliation
|
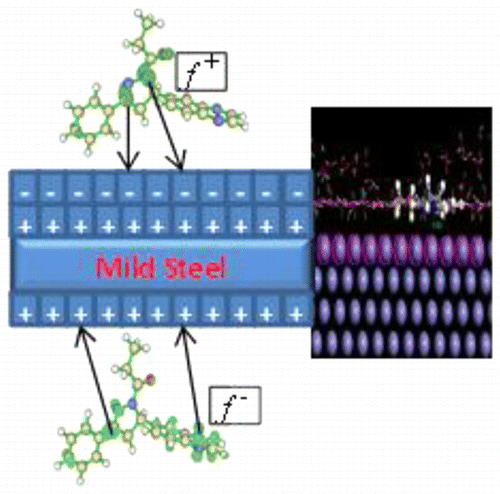
The inhibition of mild steel corrosion in 1 M HCl by some quinoxalin-6-yl derivatives namely 1-[3-phenyl-5-quinoxalin-6-yl-4,5-dihydropyrazol-1-yl]butan-1-one (PQDPB), 1-(3-phenyl-5-(quinoxalin-6-yl)-4,5-dihydro-1H-pyrazol-1-yl)propan-1-one (PQDPP), and 2-phenyl-1-[3-phenyl-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-1-yl]ethanone (PPQDPE) has been investigated using electrochemical studies and quantum chemical calculations. The results showed that PQDPP is the best corrosion inhibitor among the three compounds studied and the inhibition efficiency increases with increase in concentration for all the inhibitors. The adsorption of inhibitor molecules on mild steel surface was found to be spontaneous and obeyed the Frumkin adsorption isotherm. Scanning electron microscopy (SEM) images confirmed the formation of protective films of the inhibitors on mild steel surface. Quantum chemical calculations showed that the inhibitors have the tendency to be protonated in the acid and the results agree with experimental observations. Monte Carlo simulations were applied to search for the most stable configuration and adsorption energy for the interaction of inhibitors on Fe(110)/100 H2O interface. The results of the Monte Carlo simulations accord with the experimentally determined inhibition efficiencies. Different carbonyl substituents on the common nucleus of the three compounds obviously contributed to the difference in inhibition efficiency.
中文翻译:

一些喹喔啉-6-基衍生物作为低碳钢在盐酸中的缓蚀剂:实验和理论研究
一些喹喔啉-6-基衍生物即1- [3-苯基-5-喹喔啉-6-基-4,5-二氢吡唑-1-基]丁-1--1-酮对1 M HCl中低碳钢腐蚀的抑制作用( PQDPB),1-(3-苯基-5-(喹喔啉-6-基)-4,5-二氢-1 H-吡唑-1-基)丙-1-酮(PQDPP)和2-苯基-1- [3-苯基-5-(喹喔啉-6-基)-4,5-二氢吡唑-1-基]乙酮( PPQDPE)已使用电化学研究和量子化学计算进行了研究。结果表明,在所研究的三种化合物中,PQDPP是最佳的缓蚀剂,并且所有缓蚀剂的浓度均随着缓蚀效率的提高而提高。发现抑制剂分子在低碳钢表面上的吸附是自发的,并遵守Frumkin吸附等温线。扫描电子显微镜(SEM)图像证实了低碳钢表面上抑制剂的保护膜的形成。量子化学计算表明该抑制剂具有在酸中质子化的趋势,其结果与实验观察结果一致。2 O接口。蒙特卡洛模拟的结果符合实验确定的抑制效率。三种化合物共同核上的不同羰基取代基显然是造成抑制效率差异的原因。
更新日期:2015-07-06
中文翻译:

一些喹喔啉-6-基衍生物作为低碳钢在盐酸中的缓蚀剂:实验和理论研究
一些喹喔啉-6-基衍生物即1- [3-苯基-5-喹喔啉-6-基-4,5-二氢吡唑-1-基]丁-1--1-酮对1 M HCl中低碳钢腐蚀的抑制作用( PQDPB),1-(3-苯基-5-(喹喔啉-6-基)-4,5-二氢-1 H-吡唑-1-基)丙-1-酮(PQDPP)和2-苯基-1- [3-苯基-5-(喹喔啉-6-基)-4,5-二氢吡唑-1-基]乙酮( PPQDPE)已使用电化学研究和量子化学计算进行了研究。结果表明,在所研究的三种化合物中,PQDPP是最佳的缓蚀剂,并且所有缓蚀剂的浓度均随着缓蚀效率的提高而提高。发现抑制剂分子在低碳钢表面上的吸附是自发的,并遵守Frumkin吸附等温线。扫描电子显微镜(SEM)图像证实了低碳钢表面上抑制剂的保护膜的形成。量子化学计算表明该抑制剂具有在酸中质子化的趋势,其结果与实验观察结果一致。2 O接口。蒙特卡洛模拟的结果符合实验确定的抑制效率。三种化合物共同核上的不同羰基取代基显然是造成抑制效率差异的原因。






























 京公网安备 11010802027423号
京公网安备 11010802027423号