Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microkinetic analysis of C3–C5 ketone hydrogenation over supported Ru catalysts
Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-03-25 14:35:58 Omar Ali Abdelrahman, Andreas Heyden, Jesse Q. Bond
Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-03-25 14:35:58 Omar Ali Abdelrahman, Andreas Heyden, Jesse Q. Bond
|
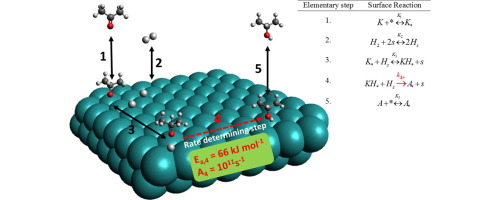
Rates of C3-C5 ketone hydrogenation are measured in the vapor phase over Ru/SiO2. Reaction kinetics are considered through a range of ketone partial pressures (0.3–30Torr), hydrogen partial pressures (90–900Torr), and reaction temperatures (322–456K). Ketone hydrogenation is observed to be facile, with site time yields ranging from 0.14s−1 for 2-pentanone to 0.37s−1 for acetone at 322K and 1.2 bar H2. At low temperatures, apparent reaction orders and kinetic barriers are similar for all ketones. During acetone hydrogenation at higher temperatures, (422K), the ketone order increases from 0 to 0.4, while the hydrogen order increases from 0.5 to 0.9. Furthermore, the apparent barrier decreases from ≈50kJmol−1 at 322K to ≈18kJmol−1. Apparent trends in hydrogenation rates are interpreted at an elementary level using a microkinetic analysis that is based on a Horiuti-Polanyi mechanism involving two distinct surface sites.
中文翻译:

负载型Ru催化剂上C3-C5酮加氢的动力学分析
在Ru / SiO 2上在气相中测量C 3 -C 5酮氢化的速率。通过一系列的酮分压(0.3–30Torr),氢分压(90–900Torr)和反应温度(322–456K)来考虑反应动力学。观察到酮氢化很容易,在322K和1.2 bar H 2下,位点时间产率范围从2-戊酮的0.14s -1到丙酮的0.37s -1。在低温下,所有酮的表观反应阶数和动力学垒均相似。在较高温度(422K)下的丙酮加氢过程中,酮级从0增加到0.4,而氢级从0.5增加到0.9。此外,明显的阻挡从≈50kJmol减小-1在322K到≈18kJmol -1。使用基于包括两个不同表面位点的Horiuti-Polanyi机理的微动力学分析,可以在基本水平上解释氢化速率的明显趋势。
更新日期:2017-03-26
中文翻译:

负载型Ru催化剂上C3-C5酮加氢的动力学分析
在Ru / SiO 2上在气相中测量C 3 -C 5酮氢化的速率。通过一系列的酮分压(0.3–30Torr),氢分压(90–900Torr)和反应温度(322–456K)来考虑反应动力学。观察到酮氢化很容易,在322K和1.2 bar H 2下,位点时间产率范围从2-戊酮的0.14s -1到丙酮的0.37s -1。在低温下,所有酮的表观反应阶数和动力学垒均相似。在较高温度(422K)下的丙酮加氢过程中,酮级从0增加到0.4,而氢级从0.5增加到0.9。此外,明显的阻挡从≈50kJmol减小-1在322K到≈18kJmol -1。使用基于包括两个不同表面位点的Horiuti-Polanyi机理的微动力学分析,可以在基本水平上解释氢化速率的明显趋势。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号