当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cross‐Aldol Reaction of Isatin with Acetone Catalyzed by Leucinol: A Mechanistic Investigation
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-07-06 , DOI: 10.1002/chem.201500536 Mikhail A. Kabeshov , Ondřej Kysilka , Lubomír Rulíšek , Yury V. Suleimanov , Marco Bella , Andrei V. Malkov , Pavel Kočovský
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2015-07-06 , DOI: 10.1002/chem.201500536 Mikhail A. Kabeshov , Ondřej Kysilka , Lubomír Rulíšek , Yury V. Suleimanov , Marco Bella , Andrei V. Malkov , Pavel Kočovský
|
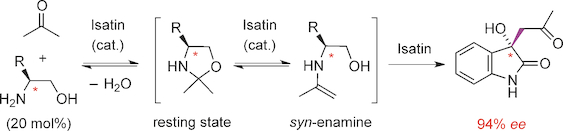
Comprehensive mechanistic studies on the enantioselective aldol reaction between isatin (1 a) and acetone, catalyzed by L‐leucinol (3 a), unraveled that isatin, apart from being a substrate, also plays an active catalytic role. Conversion of the intermediate oxazolidine 4 into the reactive syn‐enamine 6, catalyzed by isatin, was identified as the rate‐determining step by both the calculations (ΔG≠=26.1 kcal mol−1 for the analogous L‐alaninol, 3 b) and the kinetic isotope effect (kH/kD=2.7 observed for the reaction using [D6]acetone). The subsequent reaction of the syn‐enamine 6 with isatin produces (S)‐2 a (calculated ΔG≠=11.6 kcal mol−1). The calculations suggest that the overall stereochemistry is controlled by two key events: 1) the isatin‐catalyzed formation of the syn‐enamine 6, which is thermodynamically favored over its anti‐rotamer 7 by 2.3 kcal mol−1; and 2) the high preference of the syn‐enamine 6 to produce (S)‐2 a on reaction with isatin (1 a) rather than its enantiomer (ΔΔG≠=2.6 kcal mol−1).
中文翻译:

芥子醇催化的Isatin与丙酮的交叉Aldol反应:机理研究
上靛红(之间的对映选择性醛醇缩合反应的综合机理研究1)和丙酮,用催化的大号-leucinol(3),该拆散靛红,除了是一基板,也起着积极的促进作用。中间恶唑烷的转化4到反应性顺式-烯胺6,由靛红催化,被确定为所述速率确定步骤通过两者的计算(Δ ģ ≠ = 26.1千卡摩尔-1的类似大号-alaninol,图3b)以及动力学同位素效应(k H / k D使用[D 6 ]丙酮观察到= 2.7的反应。合成烯胺6与靛红的后续反应产生(S)-2 a(计算得出的ΔG ≠ = 11.6 kcal mol -1)。计算表明,整体立体化学受两个关键事件控制:1)合成酶催化的合成烯胺6的形成,在热力学上胜过其抗旋转异构体7的量为2.3 kcal mol -1;和2)合成烯胺6对生产(S) - 2上反应与靛红(1),而不是它的对映体(ΔΔ ģ ≠ = 2.6千卡摩尔-1)。
更新日期:2015-07-06
中文翻译:

芥子醇催化的Isatin与丙酮的交叉Aldol反应:机理研究
上靛红(之间的对映选择性醛醇缩合反应的综合机理研究1)和丙酮,用催化的大号-leucinol(3),该拆散靛红,除了是一基板,也起着积极的促进作用。中间恶唑烷的转化4到反应性顺式-烯胺6,由靛红催化,被确定为所述速率确定步骤通过两者的计算(Δ ģ ≠ = 26.1千卡摩尔-1的类似大号-alaninol,图3b)以及动力学同位素效应(k H / k D使用[D 6 ]丙酮观察到= 2.7的反应。合成烯胺6与靛红的后续反应产生(S)-2 a(计算得出的ΔG ≠ = 11.6 kcal mol -1)。计算表明,整体立体化学受两个关键事件控制:1)合成酶催化的合成烯胺6的形成,在热力学上胜过其抗旋转异构体7的量为2.3 kcal mol -1;和2)合成烯胺6对生产(S) - 2上反应与靛红(1),而不是它的对映体(ΔΔ ģ ≠ = 2.6千卡摩尔-1)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号