Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel non-noble bimetallic Cu-Zn/TiO2 catalysts for selective hydrogenation of butadiene
Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-03-21 14:30:40 Zhao Wang, Guillaume Wang, Catherine Louis, Laurent Delannoy
Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-03-21 14:30:40 Zhao Wang, Guillaume Wang, Catherine Louis, Laurent Delannoy
|
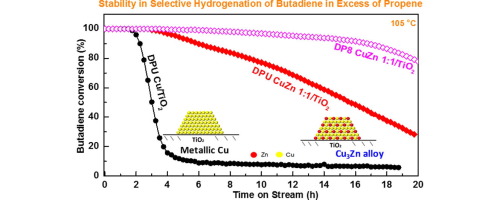
Oxide-supported copper catalysts are active for the selective hydrogenation of polyunsaturated hydrocarbons with high selectivity to alkenes formation, but a low catalytic stability limits its application. The goal of the work was to investigate whether alloying Cu with Zn will improve catalyst stability, and whether such a non-noble bimetallic catalyst could compete with noble metal-based catalysts for this type of reaction. TiO2-supported mono-metallic Cu and Zn and bimetallic Cu-Zn (Cu/Zn atomic ratio of 1 and 3) were prepared by deposition-precipitation with urea (DPU) and deposition-precipitation at pH≈8 (DP8). Small metal nanoparticles (<2nm) with uniform particle sizes were obtained from DP8 samples, while two different particle size ranges (∼3nm and >15nm) were found in the DPU samples after calcination at 400°C and then reduction at 350°C. The bimetallic character of the nanoparticles was attested by XRD and STEM-HAADF coupled with EDS analysis, as Cu3Zn alloy was found in DPU CuZn 1:1/TO2 sample and an “intermediate” Cu-Zn alloy (Cu0.9Zn0.1) was found in DPU CuZn 3:1/TiO2 sample. UV-visible spectroscopy showed that alloying of copper with zinc inhibits the reoxidation of copper by contact with air. The catalytic results showed that alloying Cu with Zn slightly decreased the activity in butadiene selective hydrogenation, but had almost no effect on the selectivity to alkenes. The important result is that the bimetallic Cu-Zn/TiO2 catalysts displayed much higher stability under isothermal reaction than Cu/TiO2, which deactivated rapidly in the first few hours. This higher stability was ascribed to the formation of a lower amount of carbonaceous species on the bimetallic catalysts, as revealed by TGA analyses. Moreover, the DP8 Cu-Zn/TiO2 samples showed lower T100% but higher stability than the corresponding DPU samples. Surprisingly, change in the composition of the Cu-Zn alloy was observed during the catalytic reaction at 105°C, with a transition from Cu3Zn to Cu0.7Zn0.3 alloy for the DPU CuZn 1:1/TiO2 sample.
中文翻译:

用于丁二烯选择性加氢的新型非贵金属双金属Cu-Zn / TiO2催化剂
氧化物负载的铜催化剂对多不饱和烃的选择性加氢具有很高的活性,对烯烃的形成具有高选择性,但是低的催化稳定性限制了其应用。这项工作的目的是研究将铜与锌合金化是否会提高催化剂的稳定性,以及这种非贵金属双金属催化剂是否可以与贵金属基催化剂竞争这种类型的反应。二氧化钛2通过尿素(DPU)的沉淀沉淀和在pH≈8(DP8)的沉淀沉淀来制备负载型单金属Cu和Zn以及双金属Cu-Zn(Cu / Zn原子比为1和3)。从DP8样品中获得粒径均匀的小金属纳米颗粒(<2nm),而在400°C下煅烧然后在350°C下还原后,DPU样品中发现了两个不同的粒径范围(〜3nm和> 15nm) C。通过XRD和STEM-HAADF结合EDS分析证明了纳米粒子的双金属特性,因为在DPU CuZn 1:1 / TO 2样品和“中间” Cu-Zn合金中发现了Cu 3 Zn合金(Cu 0.9 Zn 0.1)被发现在DPU CuZn 3:1 / TiO 2中样本。紫外可见光谱表明,铜与锌的合金化可通过与空气接触抑制铜的再氧化。催化结果表明,将铜与锌合金化会稍微降低丁二烯选择性加氢的活性,但对烯烃的选择性几乎没有影响。重要的结果是,双金属Cu-Zn / TiO 2催化剂在等温反应下表现出比Cu / TiO 2更高的稳定性,后者在最初几个小时内迅速失活。如TGA分析所揭示的,这种较高的稳定性归因于在双金属催化剂上形成较少量的碳质物质。此外,DP8 Cu-Zn / TiO 2样品的T值降低了100%但比相应的DPU样本具有更高的稳定性。令人惊讶地,在105℃的催化反应期间观察到Cu-Zn合金的组成变化,对于DPU CuZn 1:1 / TiO 2样品,从Cu 3 Zn过渡到Cu 0.7 Zn 0.3合金。
更新日期:2017-03-22
中文翻译:

用于丁二烯选择性加氢的新型非贵金属双金属Cu-Zn / TiO2催化剂
氧化物负载的铜催化剂对多不饱和烃的选择性加氢具有很高的活性,对烯烃的形成具有高选择性,但是低的催化稳定性限制了其应用。这项工作的目的是研究将铜与锌合金化是否会提高催化剂的稳定性,以及这种非贵金属双金属催化剂是否可以与贵金属基催化剂竞争这种类型的反应。二氧化钛2通过尿素(DPU)的沉淀沉淀和在pH≈8(DP8)的沉淀沉淀来制备负载型单金属Cu和Zn以及双金属Cu-Zn(Cu / Zn原子比为1和3)。从DP8样品中获得粒径均匀的小金属纳米颗粒(<2nm),而在400°C下煅烧然后在350°C下还原后,DPU样品中发现了两个不同的粒径范围(〜3nm和> 15nm) C。通过XRD和STEM-HAADF结合EDS分析证明了纳米粒子的双金属特性,因为在DPU CuZn 1:1 / TO 2样品和“中间” Cu-Zn合金中发现了Cu 3 Zn合金(Cu 0.9 Zn 0.1)被发现在DPU CuZn 3:1 / TiO 2中样本。紫外可见光谱表明,铜与锌的合金化可通过与空气接触抑制铜的再氧化。催化结果表明,将铜与锌合金化会稍微降低丁二烯选择性加氢的活性,但对烯烃的选择性几乎没有影响。重要的结果是,双金属Cu-Zn / TiO 2催化剂在等温反应下表现出比Cu / TiO 2更高的稳定性,后者在最初几个小时内迅速失活。如TGA分析所揭示的,这种较高的稳定性归因于在双金属催化剂上形成较少量的碳质物质。此外,DP8 Cu-Zn / TiO 2样品的T值降低了100%但比相应的DPU样本具有更高的稳定性。令人惊讶地,在105℃的催化反应期间观察到Cu-Zn合金的组成变化,对于DPU CuZn 1:1 / TiO 2样品,从Cu 3 Zn过渡到Cu 0.7 Zn 0.3合金。































 京公网安备 11010802027423号
京公网安备 11010802027423号