当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-Lactam analogues of combretastatin A-4 prevent metabolic inactivation by glucuronidation in chemoresistant HT-29 colon cancer cells
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-03-25 12:00:01 Azizah M. Malebari, Lisa M. Greene, Seema M. Nathwani, Darren Fayne, Niamh M. O'Boyle, Shu Wang, Brendan Twamley, Daniela M. Zisterer, Mary J. Meegan
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-03-25 12:00:01 Azizah M. Malebari, Lisa M. Greene, Seema M. Nathwani, Darren Fayne, Niamh M. O'Boyle, Shu Wang, Brendan Twamley, Daniela M. Zisterer, Mary J. Meegan
|
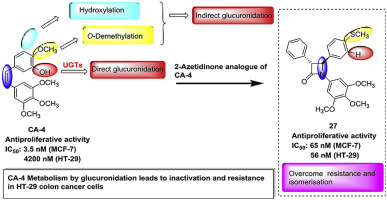
Glucuronidation by uridine 5-diphosphoglucuronosyl transferase enzymes (UGTs) is a cause of intrinsic drug resistance in cancer cells. Glucuronidation of combretastatin A-4 (CA-4) was previously identified as a mechanism of resistance in hepatocellular cancer cells. Herein, we propose chemical manipulation of β-lactam bridged analogues of Combretastatin A-4 as a novel means of overcoming drug resistance associated with glucuronidation due to the expression of UGTs in the CA-4 resistant human colon cancer HT-29 cells. The alkene bridge of CA-4 is replaced with a β-lactam ring to circumvent potential isomerisation while the potential sites of glucuronate conjugation are deleted in the novel 3-substituted-1,4-diaryl-2-azetidinone analogues of CA-4. We hypothesise that glucuronidation of CA-4 is the mechanism of drug resistance in HT-29 cells. Ring B thioether containing 2-azetidinone analogues of CA-4 such as 4-(4-(methylthio)phenyl)-3-phenyl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (27) and 3-hydroxy-4-(4-(methylthio)phenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (45) were identified as the most potent inhibitors of tumour cell growth, independent of UGT status, displaying antiproliferative activity in the low nanomolar range. These compounds also disrupted the microtubular structure in MCF-7 and HT-29 cells, and caused G2/M arrest and apoptosis. Taken together, these findings highlight the potential of chemical manipulation as a means of overcoming glucuronidation attributed drug resistance in CA-4 resistant human colon cancer HT-29 cells, allowing the development of therapeutically superior analogues.
中文翻译:

康普他汀A-4的β-内酰胺类似物可通过葡萄糖醛酸苷化预防耐化学性HT-29结肠癌细胞中的代谢失活
尿苷5-二磷酸葡糖醛酸糖基转移酶(UGT)的葡萄糖醛酸化作用是癌细胞内在抗药性的原因。康普他汀A-4(CA-4)的葡萄糖醛酸化以前被鉴定为肝细胞癌细胞耐药的机制。本文中,我们提议化学操作康布雷他汀A-4的β-内酰胺桥连类似物,作为克服由于CA-4抗性人结肠癌HT-29细胞中UGT的表达而引起的与葡萄糖醛酸化相关的耐药性的新手段。CA-4的烯烃桥被β-内酰胺环取代,以规避潜在的异构化,而在CA-4的新型3-取代的1,4-二芳基-2-氮杂环丁酮类似物中,葡萄糖醛酸共轭的潜在位点被删除。我们假设CA-4的葡萄糖醛酸化是HT-29细胞耐药的机制。含有CA-4的2-氮杂环丁酮类似物的B环硫醚,例如4-(4-(甲硫基)苯基)-3-苯基-1-(3,4,5-三甲氧基苯基)氮杂环丁酮-2-一(27)和3 -羟基-4-(4-(甲硫基)苯基)-1-(3,4,5-三甲氧基苯基)氮杂环丁烷-2-酮(45)被确定为最有效的肿瘤细胞生长抑制剂,与UGT状态无关,在低纳摩尔范围内显示出抗增殖活性。这些化合物还破坏了MCF-7和HT-29细胞的微管结构,并导致G 在低纳摩尔范围内显示出抗增殖活性。这些化合物还破坏了MCF-7和HT-29细胞的微管结构,并导致G 在低纳摩尔范围内显示出抗增殖活性。这些化合物还破坏了MCF-7和HT-29细胞的微管结构,并导致G2 / M停滞和凋亡。综上所述,这些发现突出了化学操纵作为克服CA-4抗性人结肠癌HT-29细胞中归因于药物抗性的葡萄糖醛酸化的手段的潜力,从而允许开发治疗上优越的类似物。
更新日期:2017-03-26
中文翻译:

康普他汀A-4的β-内酰胺类似物可通过葡萄糖醛酸苷化预防耐化学性HT-29结肠癌细胞中的代谢失活
尿苷5-二磷酸葡糖醛酸糖基转移酶(UGT)的葡萄糖醛酸化作用是癌细胞内在抗药性的原因。康普他汀A-4(CA-4)的葡萄糖醛酸化以前被鉴定为肝细胞癌细胞耐药的机制。本文中,我们提议化学操作康布雷他汀A-4的β-内酰胺桥连类似物,作为克服由于CA-4抗性人结肠癌HT-29细胞中UGT的表达而引起的与葡萄糖醛酸化相关的耐药性的新手段。CA-4的烯烃桥被β-内酰胺环取代,以规避潜在的异构化,而在CA-4的新型3-取代的1,4-二芳基-2-氮杂环丁酮类似物中,葡萄糖醛酸共轭的潜在位点被删除。我们假设CA-4的葡萄糖醛酸化是HT-29细胞耐药的机制。含有CA-4的2-氮杂环丁酮类似物的B环硫醚,例如4-(4-(甲硫基)苯基)-3-苯基-1-(3,4,5-三甲氧基苯基)氮杂环丁酮-2-一(27)和3 -羟基-4-(4-(甲硫基)苯基)-1-(3,4,5-三甲氧基苯基)氮杂环丁烷-2-酮(45)被确定为最有效的肿瘤细胞生长抑制剂,与UGT状态无关,在低纳摩尔范围内显示出抗增殖活性。这些化合物还破坏了MCF-7和HT-29细胞的微管结构,并导致G 在低纳摩尔范围内显示出抗增殖活性。这些化合物还破坏了MCF-7和HT-29细胞的微管结构,并导致G 在低纳摩尔范围内显示出抗增殖活性。这些化合物还破坏了MCF-7和HT-29细胞的微管结构,并导致G2 / M停滞和凋亡。综上所述,这些发现突出了化学操纵作为克服CA-4抗性人结肠癌HT-29细胞中归因于药物抗性的葡萄糖醛酸化的手段的潜力,从而允许开发治疗上优越的类似物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号