当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Utilizing Selenocysteine for Expressed Protein Ligation and Bioconjugations
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-02-27 , DOI: 10.1021/jacs.6b10991 Jun Liu 1 , Qingqing Chen 1 , Sharon Rozovsky 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-02-27 , DOI: 10.1021/jacs.6b10991 Jun Liu 1 , Qingqing Chen 1 , Sharon Rozovsky 1
Affiliation
|
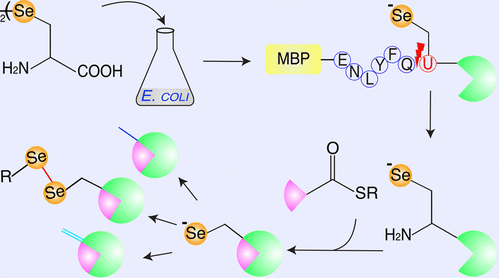
Employing selenocysteine-containing protein fragments to form the amide bond between respective protein fragments significantly extends the current capabilities of the widely used protein engineering method, expressed protein ligation. Selenocysteine-mediated ligation is noteworthy for its high yield and efficiency. However, it has so far been restricted to solid-phase synthesized seleno-peptides and thus constrained by where the selenocysteine can be positioned. Here we employ heterologously expressed seleno-fragments to overcome the placement and size restrictions in selenocysteine-mediated chemical ligation. Following ligation, the selenocysteine can be deselenized into an alanine or serine, resulting in nonselenoproteins. This greatly extends the flexibility in selecting the conjugation site in expressed protein ligations with no influence on native cysteines. Furthermore, the selenocysteine can be used to selectively introduce site-specific protein modifications. Therefore, selenocysteine-mediated expressed protein ligation simplifies incorporation of post-translational modifications into the protein scaffold.
中文翻译:

利用硒代半胱氨酸进行表达蛋白连接和生物共轭
采用含硒代半胱氨酸的蛋白质片段在各个蛋白质片段之间形成酰胺键显着扩展了目前广泛使用的蛋白质工程方法(表达蛋白质连接)的能力。硒代半胱氨酸介导的连接因其高产量和高效率而值得注意。然而,迄今为止,它仅限于固相合成的硒肽,因此受到硒代半胱氨酸的定位位置的限制。在这里,我们采用异源表达的硒片段来克服硒代半胱氨酸介导的化学连接中的位置和大小限制。连接后,硒代半胱氨酸可脱硒成丙氨酸或丝氨酸,产生非硒蛋白。这极大地扩展了表达蛋白连接中选择缀合位点的灵活性,而不影响天然半胱氨酸。此外,硒代半胱氨酸可用于选择性地引入位点特异性蛋白质修饰。因此,硒代半胱氨酸介导的表达蛋白连接简化了翻译后修饰到蛋白支架中的掺入。
更新日期:2017-02-27
中文翻译:

利用硒代半胱氨酸进行表达蛋白连接和生物共轭
采用含硒代半胱氨酸的蛋白质片段在各个蛋白质片段之间形成酰胺键显着扩展了目前广泛使用的蛋白质工程方法(表达蛋白质连接)的能力。硒代半胱氨酸介导的连接因其高产量和高效率而值得注意。然而,迄今为止,它仅限于固相合成的硒肽,因此受到硒代半胱氨酸的定位位置的限制。在这里,我们采用异源表达的硒片段来克服硒代半胱氨酸介导的化学连接中的位置和大小限制。连接后,硒代半胱氨酸可脱硒成丙氨酸或丝氨酸,产生非硒蛋白。这极大地扩展了表达蛋白连接中选择缀合位点的灵活性,而不影响天然半胱氨酸。此外,硒代半胱氨酸可用于选择性地引入位点特异性蛋白质修饰。因此,硒代半胱氨酸介导的表达蛋白连接简化了翻译后修饰到蛋白支架中的掺入。































 京公网安备 11010802027423号
京公网安备 11010802027423号