当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Friedel–Crafts C−H Borylation of Electron‐Rich Arenes: Dramatic Rate Acceleration by Added Alkenes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-02-28 , DOI: 10.1002/anie.201611536 Qin Yin 1 , Hendrik F. T. Klare 1 , Martin Oestreich 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-02-28 , DOI: 10.1002/anie.201611536 Qin Yin 1 , Hendrik F. T. Klare 1 , Martin Oestreich 1
Affiliation
|
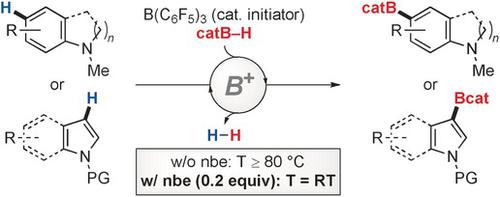
In the electrophilic C−H borylation of electron‐rich aromatic compounds with catecholborane, the catalytic generation of the boron electrophile is initiated by heterolysis of the B−H bond by various Lewis and Brønsted acids, with a boronium ion formed exclusively. After ligand dissociation, the corresponding borenium ion undergoes regioselective electrophilic aromatic substitution on aniline derivatives as well as nitrogen‐containing heterocycles. The catalysis is optimized using B(C6F5)3 as the initiator and proceeds without the addition of an external base or dihydrogen acceptor. Temperatures above 80 °C are generally required to secure efficient turnover in these Friedel–Crafts‐type reactions. Mechanistic experiments reveal that regeneration of the boronium/borenium ion with dihydrogen release is rate‐determining. This finding finally led to the discovery that, with added alkenes, catalytic C−H borylations can, for the first time, be carried out at room temperature.
中文翻译:

富电子芳烃的催化Friedel–Crafts CH硼氢化反应:增加的烯烃加速速率
在富电子芳族化合物与儿茶酚硼烷的亲电CHH硼化反应中,亲电硼的催化生成是由各种Lewis和Brønsted酸对BH键的杂化作用引发的,它仅形成硼离子。配体解离后,相应的硼离子在苯胺衍生物以及含氮杂环上进行区域选择性亲电芳香取代。使用B(C 6 F 5)3来优化催化作用作为引发剂,并且在不添加外部碱或二氢受体的情况下进行。为了确保这些Friedel-Crafts型反应的有效转换,通常需要高于80°C的温度。机理实验表明,释放氢的硼/硼离子的再生是决定速率的。这一发现最终导致人们发现,添加烯烃后,催化CHH硼化可以首次在室温下进行。
更新日期:2017-02-28
中文翻译:

富电子芳烃的催化Friedel–Crafts CH硼氢化反应:增加的烯烃加速速率
在富电子芳族化合物与儿茶酚硼烷的亲电CHH硼化反应中,亲电硼的催化生成是由各种Lewis和Brønsted酸对BH键的杂化作用引发的,它仅形成硼离子。配体解离后,相应的硼离子在苯胺衍生物以及含氮杂环上进行区域选择性亲电芳香取代。使用B(C 6 F 5)3来优化催化作用作为引发剂,并且在不添加外部碱或二氢受体的情况下进行。为了确保这些Friedel-Crafts型反应的有效转换,通常需要高于80°C的温度。机理实验表明,释放氢的硼/硼离子的再生是决定速率的。这一发现最终导致人们发现,添加烯烃后,催化CHH硼化可以首次在室温下进行。































 京公网安备 11010802027423号
京公网安备 11010802027423号