当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One- or Two-Electron Water Oxidation, Hydroxyl Radical, or H2O2 Evolution
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-02-27 00:00:00 , DOI: 10.1021/acs.jpclett.6b02924
Samira Siahrostami 1 , Guo-Ling Li 2, 3 , Venkatasubramanian Viswanathan 4 , Jens K. Nørskov 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2017-02-27 00:00:00 , DOI: 10.1021/acs.jpclett.6b02924
Samira Siahrostami 1 , Guo-Ling Li 2, 3 , Venkatasubramanian Viswanathan 4 , Jens K. Nørskov 1, 2
Affiliation
|
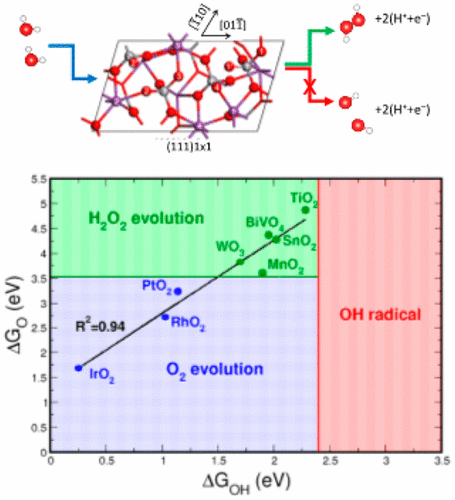
Electrochemical or photoelectrochemcial oxidation of water to form hydrogen peroxide (H2O2) or hydroxyl radicals (•OH) offers a very attractive route to water disinfection, and the first process could be the basis for a clean way to produce hydrogen peroxide. A major obstacle in the development of effective catalysts for these reactions is that the electrocatalyst must suppress the thermodynamically favored four-electron pathway leading to O2 evolution. We develop a thermochemical picture of the catalyst properties that determine selectivity toward the one, two, and four electron processes leading to •OH, H2O2, and O2.
中文翻译:

一或二电子水氧化,羟基自由基或H 2 O 2逸出
水的电化学或光电化学氧化形成过氧化氢(H 2 O 2)或羟基自由基(• OH)为水消毒提供了非常诱人的途径,第一个过程可能是清洁生产过氧化氢的基础。开发用于这些反应的有效催化剂的主要障碍是电催化剂必须抑制导致O 2析出的热力学上有利的四电子途径。我们开发了催化剂性质的热化学图,该催化剂性质决定了对导致• OH,H 2 O 2和O 2的一个,两个和四个电子过程的选择性。
更新日期:2017-02-27
中文翻译:

一或二电子水氧化,羟基自由基或H 2 O 2逸出
水的电化学或光电化学氧化形成过氧化氢(H 2 O 2)或羟基自由基(• OH)为水消毒提供了非常诱人的途径,第一个过程可能是清洁生产过氧化氢的基础。开发用于这些反应的有效催化剂的主要障碍是电催化剂必须抑制导致O 2析出的热力学上有利的四电子途径。我们开发了催化剂性质的热化学图,该催化剂性质决定了对导致• OH,H 2 O 2和O 2的一个,两个和四个电子过程的选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号