当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Directed Hydrogenation of Hydroxylated Alkene Catalyzed by Bis(phosphine)cobalt Dialkyl Complexes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-02-23 00:00:00 , DOI: 10.1021/acs.joc.7b00016
Xuelu Ma 1, 2 , Ming Lei 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-02-23 00:00:00 , DOI: 10.1021/acs.joc.7b00016
Xuelu Ma 1, 2 , Ming Lei 1
Affiliation
|
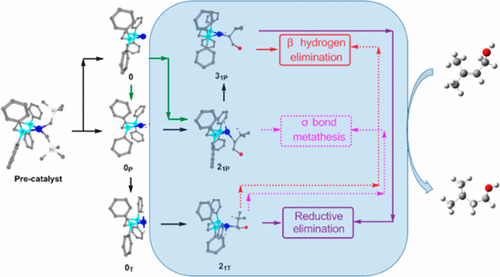
The mechanism of directed hydrogenation of hydroxylated alkene catalyzed by bis(phosphine)cobalt dialkyl complexes has been studied by DFT calculations. The possible reaction channels of alkene hydrogenation catalyzed by catalytic species (0T, 0P, and 0) were investigated. The calculated results indicate that the preferred catalytic activation processes undergo a 1,2 alkene insertion. 0P and 0 prefer the β hydrogen elimination mechanism with an energy barrier of 9.5 kcal/mol, and 0T prefers the reductive elimination mechanism with an energy barrier of 11.0 kcal/mol. The second H2 coordination in the σ bond metathesis mechanism needs to break the agostic H2–βC bond of metal–alkyl intermediates (21P and 21T), which owns the larger energetic span compared to the reductive elimination. This theoretical study shows that the most favorable reaction pathway of alkene hydrogenation is the β hydrogen elimination pathway catalyzed by the planar (dppe)CoH2. The hydrogenation activity of Co(II) compounds with redox-innocent phosphine donors involves the Co(0)–Co(II) catalytic mechanism.
中文翻译:

双(膦)钴二烷基配合物催化羟基化烯烃定向加氢的机理研究
通过DFT计算研究了双(膦)钴二烷基配合物催化羟基化烯烃的直接加氢机理。研究了0 T,0 P和0催化物种催化烯烃加氢的可能反应途径。计算结果表明,优选的催化活化过程经历了1,2个烯烃的插入。0 P和0优选具有9.5 kcal / mol的能垒的β氢消除机理,0 T优选具有11.0 kcal / mol的能垒的还原性消除机理。第二H 2σ键复分解机制中的配位需要打破金属-烷基中间体(2 1P和2 1T)的过时的H 2 -βC键,与还原消除相比,它具有更大的能量跨度。该理论研究表明,烯烃氢化最有利的反应途径是平面(dppe)CoH 2催化的β氢消除途径。Co(II)化合物与无氧化还原的膦供体的氢化活性涉及Co(0)–Co(II)催化机理。
更新日期:2017-02-23
中文翻译:

双(膦)钴二烷基配合物催化羟基化烯烃定向加氢的机理研究
通过DFT计算研究了双(膦)钴二烷基配合物催化羟基化烯烃的直接加氢机理。研究了0 T,0 P和0催化物种催化烯烃加氢的可能反应途径。计算结果表明,优选的催化活化过程经历了1,2个烯烃的插入。0 P和0优选具有9.5 kcal / mol的能垒的β氢消除机理,0 T优选具有11.0 kcal / mol的能垒的还原性消除机理。第二H 2σ键复分解机制中的配位需要打破金属-烷基中间体(2 1P和2 1T)的过时的H 2 -βC键,与还原消除相比,它具有更大的能量跨度。该理论研究表明,烯烃氢化最有利的反应途径是平面(dppe)CoH 2催化的β氢消除途径。Co(II)化合物与无氧化还原的膦供体的氢化活性涉及Co(0)–Co(II)催化机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号