当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental and Theoretical Studies on Rhodium-Catalyzed Coupling of Benzamides with 2,2-Difluorovinyl Tosylate: Diverse Synthesis of Fluorinated Heterocycles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-02-23 , DOI: 10.1021/jacs.7b00118 Jia-Qiang Wu 1 , Shang-Shi Zhang 1 , Hui Gao 1 , Zisong Qi 2 , Chu-Jun Zhou 1 , Wei-Wei Ji 1 , Yao Liu 1 , Yunyun Chen 1 , Qingjiang Li 1 , Xingwei Li 2 , Honggen Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-02-23 , DOI: 10.1021/jacs.7b00118 Jia-Qiang Wu 1 , Shang-Shi Zhang 1 , Hui Gao 1 , Zisong Qi 2 , Chu-Jun Zhou 1 , Wei-Wei Ji 1 , Yao Liu 1 , Yunyun Chen 1 , Qingjiang Li 1 , Xingwei Li 2 , Honggen Wang 1
Affiliation
|
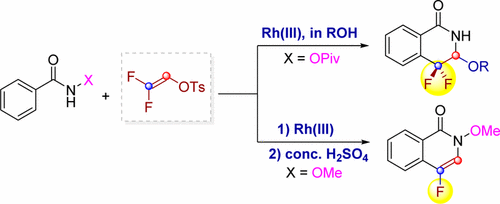
Fluorinated heterocycles play an important role in pharmaceutical and agrochemical industries. Herein, we report on the synthesis of four types of fluorinated heterocycles via rhodium(III)-catalyzed C-H activation of arenes/alkenes and versatile coupling with 2,2-difluorovinyl tosylate. With N-OMe benzamide being a directing group (DG), the reaction delivered a monofluorinated alkene with the retention of the tosylate functionality. Subsequent one-pot acid treatment allowed the efficient synthesis of 4-fluoroisoquinolin-1(2H)-ones and 5-fluoropyridin-2(1H)-ones. When N-OPiv benzamides were used, however, [4 + 2] cyclization occurred to provide gem-difluorinated dihydroisoquinolin-1(2H)-ones. Synthetic applications have been demonstrated and the ready availability of both the arene and the coupling partner highlighted the synthetic potentials of these protocols. Mechanistically, these two processes share a common process involving N-H deprotonation, C-H activation, and olefin insertion to form a 7-membered rhodacycle. Thereafter, different reaction pathways featuring β-F elimination and C-N bond formation are followed on the basis of density functional theory (DFT) studies. These two pathways are DG-dependent and led to the open chain and cyclization products, respectively. The mechanistic rationale was supported by detailed DFT studies. In particular, the origins of the intriguing selectivity in the competing β-F elimination versus C-N bond formation were elucidated. It was found that β-F elimination is a facile event and proceeds via a syn-coplanar transition state with a low energy barrier. The C-N bond formation proceeds via a facile migratory insertion of the Rh-C(alkyl) into the Rh(V) amido species. In both reactions, the migratory insertion of the alkene is turnover-limiting, which stays in good agreement with the experimental studies.
中文翻译:

苯甲酰胺与 2,2-二氟乙烯基甲苯磺酸铑催化偶联的实验和理论研究:氟化杂环的多样化合成
氟化杂环在制药和农化工业中发挥着重要作用。在此,我们报告了通过铑 (III) 催化的芳烃/烯烃的 CH 活化以及与 2,2-二氟乙烯基甲苯磺酸酯的多功能偶联来合成四种类型的氟化杂环。由于 N-OMe 苯甲酰胺是一个导向基团 (DG),该反应产生了一种单氟化烯烃,同时保留了甲苯磺酸酯官能团。随后的一锅酸处理允许有效合成 4-fluoroisoquinolin-1(2H)-ones 和 5-fluoropyridin-2(1H)-ones。然而,当使用 N-OPiv 苯甲酰胺时,会发生 [4 + 2] 环化以提供 gem-difluorinated 二氢异喹啉-1(2H)-ones。合成应用已经得到证明,芳烃和偶联伙伴的现成可用性突出了这些协议的合成潜力。从机制上讲,这两个过程共享一个共同的过程,包括 NH 去质子化、CH 活化和烯烃插入以形成 7 元罗达环。此后,在密度泛函理论(DFT)研究的基础上,遵循了以 β-F 消除和 CN 键形成为特征的不同反应途径。这两条途径是依赖于 DG 的,分别导致开链和环化产物。详细的 DFT 研究支持了机械原理。特别是,阐明了竞争性 β-F 消除与 CN 键形成中有趣的选择性的起源。发现 β-F 消除是一个轻而易举的事件,并且通过具有低能垒的共面过渡态进行。CN 键的形成是通过将 Rh-C(烷基) 轻松迁移插入 Rh(V) 酰胺类物质而进行的。在这两个反应中,烯烃的迁移插入是周转限制性的,这与实验研究保持良好的一致性。
更新日期:2017-02-23
中文翻译:

苯甲酰胺与 2,2-二氟乙烯基甲苯磺酸铑催化偶联的实验和理论研究:氟化杂环的多样化合成
氟化杂环在制药和农化工业中发挥着重要作用。在此,我们报告了通过铑 (III) 催化的芳烃/烯烃的 CH 活化以及与 2,2-二氟乙烯基甲苯磺酸酯的多功能偶联来合成四种类型的氟化杂环。由于 N-OMe 苯甲酰胺是一个导向基团 (DG),该反应产生了一种单氟化烯烃,同时保留了甲苯磺酸酯官能团。随后的一锅酸处理允许有效合成 4-fluoroisoquinolin-1(2H)-ones 和 5-fluoropyridin-2(1H)-ones。然而,当使用 N-OPiv 苯甲酰胺时,会发生 [4 + 2] 环化以提供 gem-difluorinated 二氢异喹啉-1(2H)-ones。合成应用已经得到证明,芳烃和偶联伙伴的现成可用性突出了这些协议的合成潜力。从机制上讲,这两个过程共享一个共同的过程,包括 NH 去质子化、CH 活化和烯烃插入以形成 7 元罗达环。此后,在密度泛函理论(DFT)研究的基础上,遵循了以 β-F 消除和 CN 键形成为特征的不同反应途径。这两条途径是依赖于 DG 的,分别导致开链和环化产物。详细的 DFT 研究支持了机械原理。特别是,阐明了竞争性 β-F 消除与 CN 键形成中有趣的选择性的起源。发现 β-F 消除是一个轻而易举的事件,并且通过具有低能垒的共面过渡态进行。CN 键的形成是通过将 Rh-C(烷基) 轻松迁移插入 Rh(V) 酰胺类物质而进行的。在这两个反应中,烯烃的迁移插入是周转限制性的,这与实验研究保持良好的一致性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号