当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fingerprints of Through-Bond and Through-Space Exciton and Charge π-Electron Delocalization in Linearly Extended [2.2]Paracyclophanes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-02-21 , DOI: 10.1021/jacs.6b12520 José L. Zafra 1 , Agustín Molina Ontoria 2 , Paula Mayorga Burrezo 1 , Miriam Peña-Alvarez , Marek Samoc 3 , Janusz Szeremeta 3 , Francisco J. Ramírez 1 , Matthew D. Lovander 4 , Christopher J. Droske 4 , Ted M. Pappenfus 4 , Luis Echegoyen 5 , Juan T. López Navarrete 1 , Nazario Martín 2 , Juan Casado 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-02-21 , DOI: 10.1021/jacs.6b12520 José L. Zafra 1 , Agustín Molina Ontoria 2 , Paula Mayorga Burrezo 1 , Miriam Peña-Alvarez , Marek Samoc 3 , Janusz Szeremeta 3 , Francisco J. Ramírez 1 , Matthew D. Lovander 4 , Christopher J. Droske 4 , Ted M. Pappenfus 4 , Luis Echegoyen 5 , Juan T. López Navarrete 1 , Nazario Martín 2 , Juan Casado 1
Affiliation
|
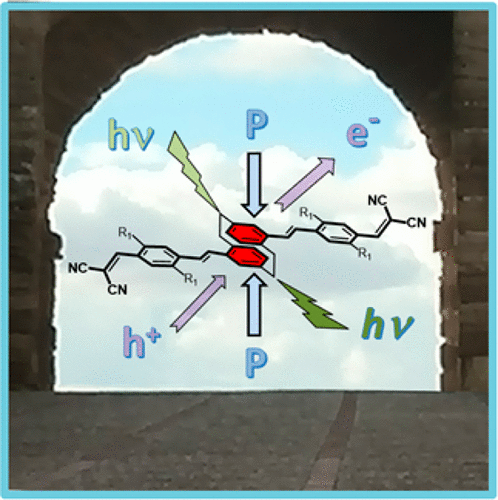
New stilbenoid and thiophenic compounds terminally functionalized with donor-donor, acceptor-acceptor, or donor-acceptor moieties and possessing a central [2.2]paracyclophane unit have been prepared, and their properties interpreted in terms of through-bond and through space π-electron delocalization (i.e., π-conjugations). Based on photophysical data, their excited-state properties have been described with a focus on the participation of the central [2.2]paracyclophane in competition with through-bond conjugation in the side arms. To this end, two-photon and one-photon absorption and emission spectroscopy, as a function of temperature, solvent polarity, and pressure in the solid state have been recorded. Furthermore, charge delocalization through the [2.2]paracyclophane in the neutral state and in the oxidized species (radical cations, dications and radical trications) has been investigated, allowing the elucidation of the vibrational Raman fingerprint of through-space charge delocalization. Thus, a complementary approach to both "intermolecular" excitation and charge delocalizations in [2.2]paracyclophane molecules is shown which can serve as models of charge and exciton migration in organic semiconductors.
中文翻译:

线性扩展 [2.2] 对环芳烃中的贯穿键和贯穿空间激子和电荷 π 电子离域的指纹
已经制备了具有供体-供体、受体-受体或供体-受体部分末端官能化并具有中心 [2.2] 对环烷单元的新型芪类和噻吩化合物,并根据通过键和通过空间 π 电子解释了它们的性质离域化(即 π 共轭)。基于光物理数据,它们的激发态特性已被描述,重点是中心 [2.2] 对环芳烷参与与侧臂中的键共轭竞争。为此,已经记录了作为温度、溶剂极性和固态压力的函数的双光子和单光子吸收和发射光谱。此外,通过 [2.2] 对环烷在中性状态和氧化物质(自由基阳离子,dications 和激进的 trications) 已经被研究,允许阐明通过空间电荷离域的振动拉曼指纹。因此,显示了 [2.2] 对环芳烷分子中“分子间”激发和电荷离域的互补方法,可用作有机半导体中电荷和激子迁移的模型。
更新日期:2017-02-21
中文翻译:

线性扩展 [2.2] 对环芳烃中的贯穿键和贯穿空间激子和电荷 π 电子离域的指纹
已经制备了具有供体-供体、受体-受体或供体-受体部分末端官能化并具有中心 [2.2] 对环烷单元的新型芪类和噻吩化合物,并根据通过键和通过空间 π 电子解释了它们的性质离域化(即 π 共轭)。基于光物理数据,它们的激发态特性已被描述,重点是中心 [2.2] 对环芳烷参与与侧臂中的键共轭竞争。为此,已经记录了作为温度、溶剂极性和固态压力的函数的双光子和单光子吸收和发射光谱。此外,通过 [2.2] 对环烷在中性状态和氧化物质(自由基阳离子,dications 和激进的 trications) 已经被研究,允许阐明通过空间电荷离域的振动拉曼指纹。因此,显示了 [2.2] 对环芳烷分子中“分子间”激发和电荷离域的互补方法,可用作有机半导体中电荷和激子迁移的模型。

































 京公网安备 11010802027423号
京公网安备 11010802027423号