当前位置:
X-MOL 学术
›
Dyes Pigments
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of planarized intramolecular charge-transfer state in dichlorotriazinyl-pyrene fluorescent probe: TD-DFT and resonance Raman study
Dyes and Pigments ( IF 4.1 ) Pub Date : 2017-03-11 06:44:00 Tomáš Staněk, Miroslav Dvořák, Numan Almonasy, Miloš Nepraš, Ivana Šloufová, Martin Michl
Dyes and Pigments ( IF 4.1 ) Pub Date : 2017-03-11 06:44:00 Tomáš Staněk, Miroslav Dvořák, Numan Almonasy, Miloš Nepraš, Ivana Šloufová, Martin Michl
|
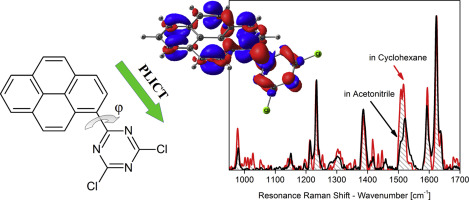
This study is focused on explanation of the remarkable photophysical behaviour of the 1-(4,6-dichloro-1,3,5-triazin-2-yl)-pyrene (PyTC2) compound which has been introduced as a fluorescent polarity probe. This compound exhibits large solvatochromic red-shift of fluorescence emission band while maintaining high fluorescence quantum yield and monoexponential decay kinetics throughout the whole solvent polarity scale. As the semi-empirical calculations reported in the original paper have not revealed any excited state possessing a high dipole moment, it has been suggested that the red-shift originates from planarization of the emitting excited state in polar solvents in contrast to unchanged twisted geometry in non-polar solvents. However, both the extent of the red-shift and the disappearance of the vibronic structure in polar solvents indicate that the emission originates from an excited state with high dipole moment and that the semi-empirical methods may not be sufficient to describe the emitting state of this molecule correctly. Thus, we have performed TD-DFT calculations including the potential energy surface scans. According to these calculations and scans, the emission takes place from a planarized intramolecular charge-transfer (ICT) state. This is in good agreement with all aspects of the observed fluorescence behaviour of PyTC2. Independent experimental evidence for the ICT has been provided by analysis of resonance Raman intensities where bands corresponding to enhanced normal modes residing on triazine and the stretching mode between pyrene and triazine moieties have been identified. The formation of the photoinduced ICT together with easy and inexpensive preparation make this compound and its derivatives candidate as push-pull building blocks for the design of advanced functional materials.
中文翻译:

二氯三嗪基-荧光探针中平面化分子内电荷转移态的形成:TD-DFT和共振拉曼研究
这项研究集中于解释1-(4,6-dichloro-1,3,5-triazin-2-yl)-(PyTC2)化合物的显着光物理行为,该化合物已作为荧光极性探针引入。该化合物在整个溶剂极性范围内保持高荧光量子产率和单指数衰减动力学的同时,在荧光发射带上表现出较大的溶剂变色红移。由于原始论文中报道的半经验计算未揭示任何具有高偶极矩的激发态,因此有人提出,红移源自极性溶剂中发射激发态的平面化,与之相对的是扭曲的几何形状。非极性溶剂。然而,极性溶剂中红移的程度和振子结构的消失均表明发射源自具有高偶极矩的激发态,并且半经验方法可能不足以描述该分子的发射态正确地。因此,我们执行了TD-DFT计算,包括势能面扫描。根据这些计算和扫描,发射从平面化的分子内电荷转移(ICT)状态发生。这与观察到的PyTC2荧光行为的所有方面都非常吻合。通过对共振拉曼强度的分析,为ICT提供了独立的实验证据,其中已经确定了与驻留在三嗪上的增强正常模式以及pyr与三嗪部分之间的拉伸模式相对应的谱带。光诱导ICT的形成以及简便且廉价的制备方法,使得该化合物及其衍生物成为了设计高级功能材料的推挽结构基石。
更新日期:2017-03-12
中文翻译:

二氯三嗪基-荧光探针中平面化分子内电荷转移态的形成:TD-DFT和共振拉曼研究
这项研究集中于解释1-(4,6-dichloro-1,3,5-triazin-2-yl)-(PyTC2)化合物的显着光物理行为,该化合物已作为荧光极性探针引入。该化合物在整个溶剂极性范围内保持高荧光量子产率和单指数衰减动力学的同时,在荧光发射带上表现出较大的溶剂变色红移。由于原始论文中报道的半经验计算未揭示任何具有高偶极矩的激发态,因此有人提出,红移源自极性溶剂中发射激发态的平面化,与之相对的是扭曲的几何形状。非极性溶剂。然而,极性溶剂中红移的程度和振子结构的消失均表明发射源自具有高偶极矩的激发态,并且半经验方法可能不足以描述该分子的发射态正确地。因此,我们执行了TD-DFT计算,包括势能面扫描。根据这些计算和扫描,发射从平面化的分子内电荷转移(ICT)状态发生。这与观察到的PyTC2荧光行为的所有方面都非常吻合。通过对共振拉曼强度的分析,为ICT提供了独立的实验证据,其中已经确定了与驻留在三嗪上的增强正常模式以及pyr与三嗪部分之间的拉伸模式相对应的谱带。光诱导ICT的形成以及简便且廉价的制备方法,使得该化合物及其衍生物成为了设计高级功能材料的推挽结构基石。

































 京公网安备 11010802027423号
京公网安备 11010802027423号