当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First Stereoselective Total Synthesis of a Dimeric Naphthoquinonopyrano‐γ‐lactone: (+)‐γ‐Actinorhodin
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-02-17 , DOI: 10.1002/anie.201611183 Markus Neumeyer 1 , Reinhard Brückner 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-02-17 , DOI: 10.1002/anie.201611183 Markus Neumeyer 1 , Reinhard Brückner 1
Affiliation
|
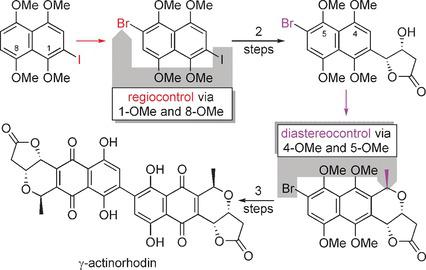
We have accomplished the first total synthesis of an isomerically pure naphthoquinonopyrano‐γ‐lactone dimer, γ‐actinorhodin, in eleven steps. Two steps exploit pairs of peri‐MeO groups as unusual selectivity controls. The respective MeO groups convey the steric bulk of a bromo or iodo substituent located ortho to one MeO group as steric hindrance into the vicinity of the second MeO group. This relay effect was indispensable for exerting regiocontrol in an aromatic bromination and diastereocontrol in an oxa‐Pictet–Spengler cyclization. The absolute configuration of our target compound was established in an asymmetric Sharpless dihydroxylation of a β,γ‐unsaturated ester, which was synthesized in a Heck coupling of a bromoiodonaphthalene with ethyl vinylacetate. The dihydroxylation provided the γ‐hydroxylactone moiety of the bromonaphthalene that was used as the substrate in the oxa‐Pictet–Spengler cyclization. Dimerization to the core of γ‐actinorhodin occurred by two Suzuki couplings.
中文翻译:

二聚萘醌喹吡喃-γ-内酯的立体选择性全合成:(+)-γ-放线菌丝蛋白
我们已经通过11个步骤完成了一个异构体纯的萘醌对吡喃酮-γ-内酯二聚体γ-肌动蛋白的首次全合成。分两步将成对的MeO-MeO基团用作异常的选择性对照。各个MeO基团将位于一个MeO基团附近的溴或碘取代基的空间本体作为空间位阻传递到第二MeO基团的附近。这种中继效应对于在oxa-Pictet-Spengler环化反应中的芳香族溴化反应和非对映异构控制作用不可或缺。我们的目标化合物的绝对构型是由β,γ-不饱和酯的不对称Sharpless二羟基化反应建立的,该反应是通过溴碘代萘与乙烯基乙酸乙酯的Heck偶联反应合成的。二羟基化作用提供了溴萘的γ-羟基内酯部分,该残基被用作oxa-Pictet-Spengler环化反应的底物。通过两个Suzuki偶联发生向γ-放线菌丝蛋白核心的二聚化。
更新日期:2017-02-17
中文翻译:

二聚萘醌喹吡喃-γ-内酯的立体选择性全合成:(+)-γ-放线菌丝蛋白
我们已经通过11个步骤完成了一个异构体纯的萘醌对吡喃酮-γ-内酯二聚体γ-肌动蛋白的首次全合成。分两步将成对的MeO-MeO基团用作异常的选择性对照。各个MeO基团将位于一个MeO基团附近的溴或碘取代基的空间本体作为空间位阻传递到第二MeO基团的附近。这种中继效应对于在oxa-Pictet-Spengler环化反应中的芳香族溴化反应和非对映异构控制作用不可或缺。我们的目标化合物的绝对构型是由β,γ-不饱和酯的不对称Sharpless二羟基化反应建立的,该反应是通过溴碘代萘与乙烯基乙酸乙酯的Heck偶联反应合成的。二羟基化作用提供了溴萘的γ-羟基内酯部分,该残基被用作oxa-Pictet-Spengler环化反应的底物。通过两个Suzuki偶联发生向γ-放线菌丝蛋白核心的二聚化。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号