当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cancer cell-secreted IGF2 instigates fibroblasts and bone marrow-derived vascular progenitor cells to promote cancer progression.
Nature Communications ( IF 14.7 ) Pub Date : 2017-02-10 , DOI: 10.1038/ncomms14399
Wen Wen Xu , Bin Li , Xin Yuan Guan , Sookja K. Chung , Yang Wang , Yim Ling Yip , Simon Y. K. Law , Kin Tak Chan , Nikki P. Y. Lee , Kwok Wah Chan , Li Yan Xu , En Min Li , Sai Wah Tsao , Qing-Yu He , Annie L. M. Cheung
Nature Communications ( IF 14.7 ) Pub Date : 2017-02-10 , DOI: 10.1038/ncomms14399
Wen Wen Xu , Bin Li , Xin Yuan Guan , Sookja K. Chung , Yang Wang , Yim Ling Yip , Simon Y. K. Law , Kin Tak Chan , Nikki P. Y. Lee , Kwok Wah Chan , Li Yan Xu , En Min Li , Sai Wah Tsao , Qing-Yu He , Annie L. M. Cheung
|
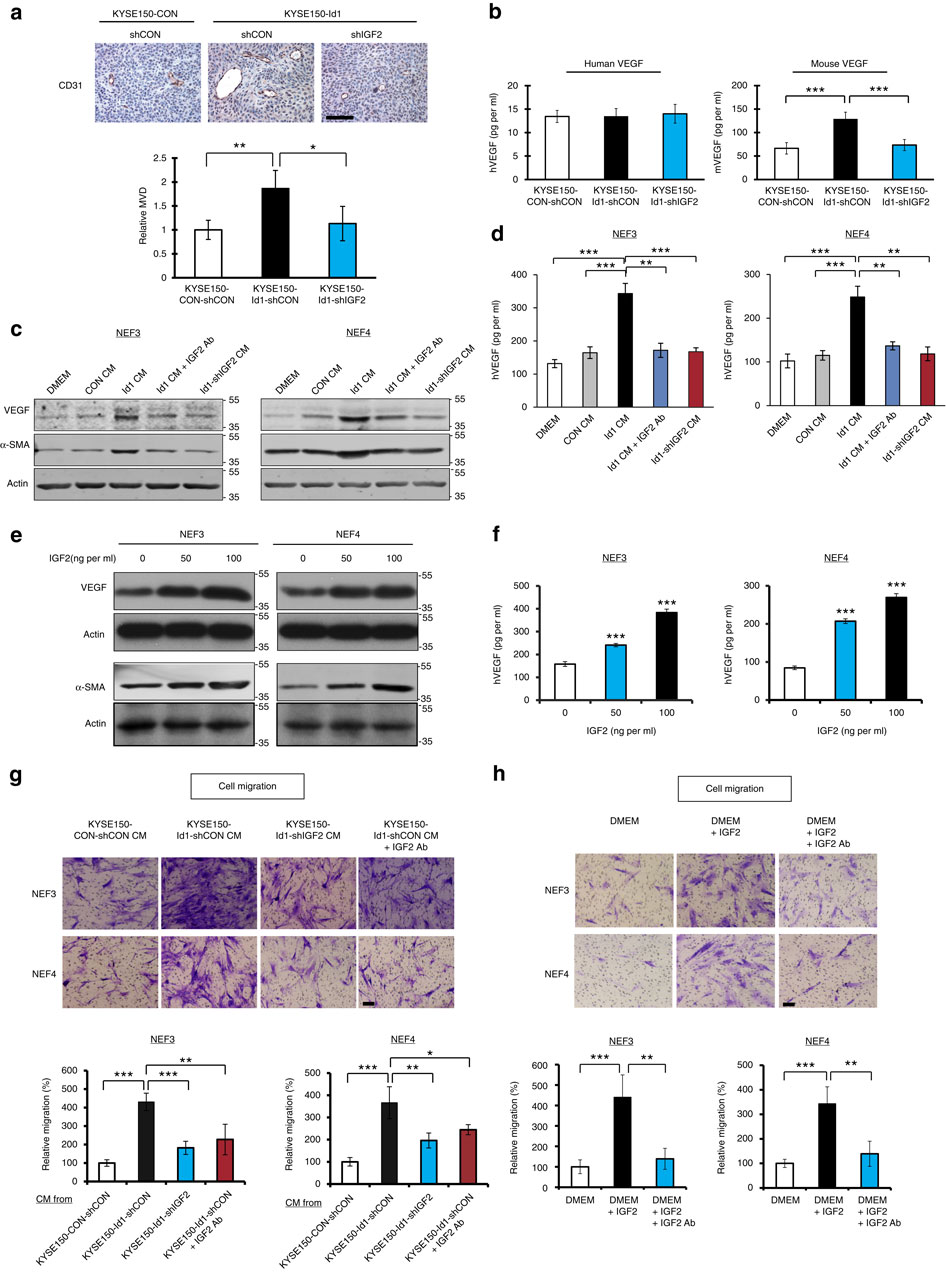
Local interactions between cancer cells and stroma can produce systemic effects on distant organs to govern cancer progression. Here we show that IGF2 secreted by inhibitor of differentiation (Id1)-overexpressing oesophageal cancer cells instigates VEGFR1-positive bone marrow cells in the tumour macroenvironment to form pre-metastatic niches at distant sites by increasing VEGF secretion from cancer-associated fibroblasts. Cancer cells are then attracted to the metastatic site via the CXCL5/CXCR2 axis. Bone marrow cells transplanted from nude mice bearing Id1-overexpressing oesophageal tumours enhance tumour growth and metastasis in recipient mice, whereas systemic administration of VEGFR1 antibody abrogates these effects. Mechanistically, IGF2 regulates VEGF in fibroblasts via miR-29c in a p53-dependent manner. Analysis of patient serum samples showed that concurrent elevation of IGF2 and VEGF levels may serve as a prognostic biomarker for oesophageal cancer. These findings suggest that the Id1/IGF2/VEGF/VEGFR1 cascade plays a critical role in tumour-driven pathophysiological processes underlying cancer progression.
中文翻译:

癌细胞分泌的IGF2促进成纤维细胞和骨髓来源的血管祖细胞促进癌症进展。
癌细胞与基质之间的局部相互作用可以对远处的器官产生全身性影响,从而控制癌症的进展。在这里,我们显示了由分化抑制因子(Id1)过表达的食道癌细胞分泌的IGF2会通过增加与癌症相关的成纤维细胞的VEGF分泌而在肿瘤大环境中刺激VEGFR1阳性骨髓细胞在远处形成转移前的壁ni。然后癌细胞通过CXCL5 / CXCR2轴被吸引到转移部位。从携带Id1过表达食道肿瘤的裸鼠移植的骨髓细胞可增强受体小鼠的肿瘤生长和转移,而全身性给予VEGFR1抗体则可消除这些作用。从机制上讲,IGF2通过miR-29c以p53依赖的方式调节成纤维细胞中的VEGF。对患者血清样本的分析表明,同时升高的IGF2和VEGF水平可能是食管癌的预后生物标志物。这些发现表明,Id1 / IGF2 / VEGF / VEGFR1级联在肿瘤发展所基于的肿瘤驱动的病理生理过程中起着至关重要的作用。
更新日期:2017-02-12
中文翻译:

癌细胞分泌的IGF2促进成纤维细胞和骨髓来源的血管祖细胞促进癌症进展。
癌细胞与基质之间的局部相互作用可以对远处的器官产生全身性影响,从而控制癌症的进展。在这里,我们显示了由分化抑制因子(Id1)过表达的食道癌细胞分泌的IGF2会通过增加与癌症相关的成纤维细胞的VEGF分泌而在肿瘤大环境中刺激VEGFR1阳性骨髓细胞在远处形成转移前的壁ni。然后癌细胞通过CXCL5 / CXCR2轴被吸引到转移部位。从携带Id1过表达食道肿瘤的裸鼠移植的骨髓细胞可增强受体小鼠的肿瘤生长和转移,而全身性给予VEGFR1抗体则可消除这些作用。从机制上讲,IGF2通过miR-29c以p53依赖的方式调节成纤维细胞中的VEGF。对患者血清样本的分析表明,同时升高的IGF2和VEGF水平可能是食管癌的预后生物标志物。这些发现表明,Id1 / IGF2 / VEGF / VEGFR1级联在肿瘤发展所基于的肿瘤驱动的病理生理过程中起着至关重要的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号