当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Buta-1,3-diyne-Based π-Conjugated Polymers for Organic Transistors and Solar Cells
Macromolecules ( IF 5.1 ) Pub Date : 2017-02-09 00:00:00 , DOI: 10.1021/acs.macromol.6b02702 Brian J. Eckstein , Ferdinand S. Melkonyan , Nanjia Zhou , Eric F. Manley 1 , Jeremy Smith , Amod Timalsina , Robert P. H. Chang , Lin X. Chen 1 , Antonio Facchetti 2 , Tobin J. Marks
Macromolecules ( IF 5.1 ) Pub Date : 2017-02-09 00:00:00 , DOI: 10.1021/acs.macromol.6b02702 Brian J. Eckstein , Ferdinand S. Melkonyan , Nanjia Zhou , Eric F. Manley 1 , Jeremy Smith , Amod Timalsina , Robert P. H. Chang , Lin X. Chen 1 , Antonio Facchetti 2 , Tobin J. Marks
Affiliation
|
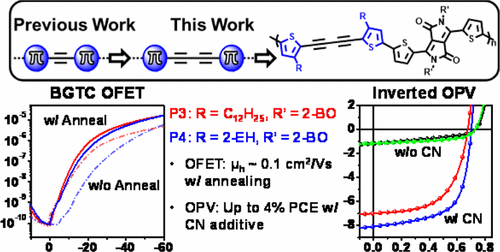
We report the synthesis and characterization of new alkyl-substituted 1,4-di(thiophen-2-yl)buta-1,3-diyne (R-DTB) donor building blocks, based on the −C≡C–C≡C– conjugative pathway, and their incorporation with thienyl-diketopyrrolopyrrole (R′-TDPP) acceptor units into π-conjugated PTDPP-DTB polymers (P1–P4). The solubility of the new polymers strongly depends on the DTB and DPP solubilizing (R and R′, respectively) substituents. Thus, solution processable and high molecular weight PDPP-DTB polymers are achieved for P3 (R = n-C12H25, R′ = 2-butyloctyl) and P4 (R = 2-ethylhexyl, R′ = 2-butyloctyl). Systematic studies of P3 and P4 physicochemical properties are carried using optical spectroscopy, cyclic voltammetry, and thermal analysis, revealing characteristic features of the dialkynyl motif. For the first time, optoelectronic devices (OFETs, OPVs) are fabricated with 1,3-butadiyne containing organic semiconductors. OFET hole mobilities and record OPV power conversion efficiencies for acetylenic organic materials approach 0.1 cm2/(V s) and 4%, respectively, which can be understood from detailed thin-film morphology and microstructural characterization using AFM, TEM, XRD, and GIWAXS methodologies. Importantly, DTB-based polymers (P3 and P4) exhibit, in addition to stabilization of frontier molecular orbitals and to −C≡C–C≡C– relief of steric torsions, discrete morphological pliability through thermal annealing and processing additives. The advantageous materials properties and preliminary device performance reported here demonstrate the promise of 1,3-butadiyne-based semiconducting polymers.
中文翻译:

基于Buta-1,3-diyne的π共轭聚合物,用于有机晶体管和太阳能电池
我们基于-C≡C-C≡C报告了新的烷基取代的1,4-二(噻吩-2-基)buta-1,3-二炔(R-DTB)供体结构单元的合成和表征-共轭途径,以及它们与噻吩基二酮吡咯并吡咯(R'-TDPP)受体单元到π共轭PTDPP-DTB聚合物掺入(P1 - P4)。新聚合物的溶解度在很大程度上取决于DTB和DPP的增溶(分别为R和R')取代基。因此,对于P3(R =n- C 12 H 25,R′= 2-丁基辛基)和P4(R = 2-乙基己基,R′= 2-丁基辛基)获得了可溶液加工的高分子量PDPP-DTB聚合物。对P3和P3的系统研究P4的理化性质通过光谱学,循环伏安法和热分析进行,揭示了二炔基基序的特征。首次使用包含1,3-丁二炔的有机半导体制造光电器件(OFET,OPV)。乙炔有机材料的OFET空穴迁移率和记录的OPV功率转换效率分别接近0.1 cm 2 /(V s)和4%,这可以通过使用AFM,TEM,XRD和GIWAXS进行的详细薄膜形态学和微观结构表征来理解方法论。重要的是,基于DTB的聚合物(P3和P4)除了具有前沿分子轨道的稳定性和–C −C–C≡C–空间扭曲的缓解以外,还具有通过热退火和加工添加剂的离散形态柔韧性。此处报道的有利的材料性能和初步的设备性能证明了基于1,3-丁二炔的半导体聚合物的前景。
更新日期:2017-02-09
中文翻译:

基于Buta-1,3-diyne的π共轭聚合物,用于有机晶体管和太阳能电池
我们基于-C≡C-C≡C报告了新的烷基取代的1,4-二(噻吩-2-基)buta-1,3-二炔(R-DTB)供体结构单元的合成和表征-共轭途径,以及它们与噻吩基二酮吡咯并吡咯(R'-TDPP)受体单元到π共轭PTDPP-DTB聚合物掺入(P1 - P4)。新聚合物的溶解度在很大程度上取决于DTB和DPP的增溶(分别为R和R')取代基。因此,对于P3(R =n- C 12 H 25,R′= 2-丁基辛基)和P4(R = 2-乙基己基,R′= 2-丁基辛基)获得了可溶液加工的高分子量PDPP-DTB聚合物。对P3和P3的系统研究P4的理化性质通过光谱学,循环伏安法和热分析进行,揭示了二炔基基序的特征。首次使用包含1,3-丁二炔的有机半导体制造光电器件(OFET,OPV)。乙炔有机材料的OFET空穴迁移率和记录的OPV功率转换效率分别接近0.1 cm 2 /(V s)和4%,这可以通过使用AFM,TEM,XRD和GIWAXS进行的详细薄膜形态学和微观结构表征来理解方法论。重要的是,基于DTB的聚合物(P3和P4)除了具有前沿分子轨道的稳定性和–C −C–C≡C–空间扭曲的缓解以外,还具有通过热退火和加工添加剂的离散形态柔韧性。此处报道的有利的材料性能和初步的设备性能证明了基于1,3-丁二炔的半导体聚合物的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号