当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced Gold-Catalyzed Domino C(sp) Arylation/Oxyarylation of TMS-Terminated Alkynols with Arenediazonium Salts
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-02-09 00:00:00 , DOI: 10.1021/acs.joc.6b03006 Benito Alcaide 1 , Pedro Almendros 2 , Eduardo Busto 1 , Carlos Lázaro-Milla 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-02-09 00:00:00 , DOI: 10.1021/acs.joc.6b03006 Benito Alcaide 1 , Pedro Almendros 2 , Eduardo Busto 1 , Carlos Lázaro-Milla 1
Affiliation
|
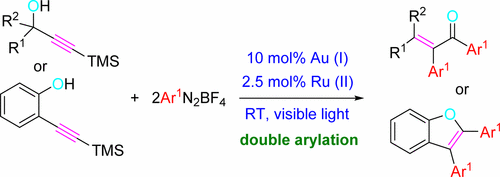
A selective and convenient synthesis of tri- and tetrasubstituted α,β-unsaturated ketones, as well as 2,3-diarylbenzofurans has been developed with the aid of light and taking advantage of a cooperative gold/photoredox-catalyzed 2-fold arylation reaction of TMS-terminated alkynols. The reaction of 3-(trimethylsilyl)prop-2-yn-1-ols was competent to generate diarylated α,β-unsaturated ketones; whereas the photoredox sequence involving 2-[(trimethylsilyl)ethynyl]phenol exclusively afforded 2,3-diarylbenzofurans. The reaction of terminal alkynes proceeded in poor yields while the use of bulkier silyl groups, such as TIPS, resulted unproductive. Apparently, the C(sp) arylation reaction is the first event on the domino bis-arylative sequence. These results could be explained through the intermediation of arylgold(III) species and several single electron transfer processes.
中文翻译:

TMS封端的炔醇与Arenediazonium盐的光诱导金催化的Domino C(sp)的芳构化/羰基化
利用光/金的协同金/光氧化还原催化的2-倍芳基化反应,开发了选择性和方便地合成三和四取代的α,β-不饱和酮以及2,3-二芳基苯并呋喃的方法。 TMS终止的炔醇。3-(三甲基甲硅烷基)丙-2-yn-1-醇的反应能生成二芳基化的α,β-不饱和酮。而涉及2-[((三甲基甲硅烷基)乙炔基]苯酚的光氧化还原序列仅提供2,3-二芳基苯并呋喃。末端炔烃的反应收率很低,而使用较大的甲硅烷基(如TIPS)则无济于事。显然,C(sp)芳基化反应是多米诺骨牌双芳基序列上的第一个事件。
更新日期:2017-02-09
中文翻译:

TMS封端的炔醇与Arenediazonium盐的光诱导金催化的Domino C(sp)的芳构化/羰基化
利用光/金的协同金/光氧化还原催化的2-倍芳基化反应,开发了选择性和方便地合成三和四取代的α,β-不饱和酮以及2,3-二芳基苯并呋喃的方法。 TMS终止的炔醇。3-(三甲基甲硅烷基)丙-2-yn-1-醇的反应能生成二芳基化的α,β-不饱和酮。而涉及2-[((三甲基甲硅烷基)乙炔基]苯酚的光氧化还原序列仅提供2,3-二芳基苯并呋喃。末端炔烃的反应收率很低,而使用较大的甲硅烷基(如TIPS)则无济于事。显然,C(sp)芳基化反应是多米诺骨牌双芳基序列上的第一个事件。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号