当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2O2-Depleting and O2-Generating Selenium Nanoparticles for Fluorescence Imaging and Photodynamic Treatment of Proinflammatory-Activated Macrophages
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-02-01 00:00:00 , DOI: 10.1021/acsami.6b15515 Kun-Ying Lu,Po-Yen Lin,Er-Yuan Chuang,Chwen-Ming Shih,Tsai-Mu Cheng,Tsung-Yao Lin,Hsing-Wen Sung,Fwu-Long Mi
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-02-01 00:00:00 , DOI: 10.1021/acsami.6b15515 Kun-Ying Lu,Po-Yen Lin,Er-Yuan Chuang,Chwen-Ming Shih,Tsai-Mu Cheng,Tsung-Yao Lin,Hsing-Wen Sung,Fwu-Long Mi
|
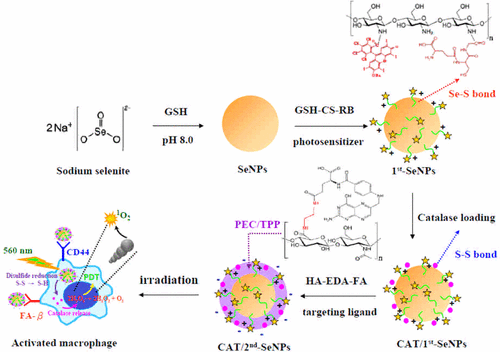
Macrophages have a pivotal role in chronic inflammatory diseases (CIDs), so imaging and controlling activated macrophage is critical for detecting and reducing chronic inflammation. In this study, photodynamic selenium nanoparticles (SeNPs) with photosensitive and macrophage-targeting bilayers were developed. The first layer of the photosensitive macromolecule was composed of a conjugate of a photosensitizer (rose bengal, RB) and a thiolated chitosan (chitosan-glutathione), resulting in a plasmonic coupling-induced red shift and broadening of RB absorption bands with increased absorption intensity. Electron paramagnetic resonance (EPR) and diphenylanthracene (DPA) quenching studies revealed that the SeNPs that were coated with the photosensitive layer were more effective than RB alone in producing singlet oxygen (1O2) under photoirradiation. The second layer of the activated macrophage-targetable macromolecule was synthesized by conjugation of hyaluronic acid with folic acid using an ethylenediamine linker. Proinflammatory-activated macrophages rapidly internalized the SeNPs that were covered with the targeting ligand, exhibiting a much stronger fluorescence signal of the SeNPs than did the nonactivated macrophages. Since proinflammatory-activated macrophage was known to generate a substantial amount of H2O2 while the inflamed site generally caused inflammation-associated tissue hypoxia, the SeNPs were further modified with O2 self-sufficient function for photodynamic therapy. Catalase was immobilized on the SeNPs by the formation of disulfide bonds. Intracellular reduction of disulfide bonds induced the subsequent release of catalase, which catalyzed the decomposition of H2O2. The H2O2-depleting and O2-generating photodynamic SeNPs efficiently killed activated macrophages and quenched the intracellular H2O2 and NO that are associated with inflammation. The SeNPs may have potential as a theranostic nanomaterial to image and control the activation of macrophages.
中文翻译:

H 2 O 2消耗和O 2生成硒纳米粒子的荧光成像和光动力治疗促炎性巨噬细胞。
巨噬细胞在慢性炎症疾病(CID)中起着关键作用,因此成像和控制活化的巨噬细胞对于检测和减少慢性炎症至关重要。在这项研究中,开发了具有光敏和巨噬细胞靶向双层的光动力学硒纳米颗粒(SeNPs)。光敏大分子的第一层由光敏剂(玫瑰红,RB)和硫醇化壳聚糖(壳聚糖-谷胱甘肽)的共轭物组成,导致等离子体耦合引起的红移和RB吸收谱带的扩展,吸收强度增加。电子顺磁共振(EPR)和二苯基蒽(DPA)淬灭研究表明,涂有光敏层的SeNP在产生单线态氧方面比单独的RB更有效(1O 2)在光照射下。通过使用乙二胺接头使透明质酸与叶酸缀合来合成活化的巨噬细胞可靶向大分子的第二层。促炎激活的巨噬细胞迅速内化了被靶向配体覆盖的SeNP,与未激活的巨噬细胞相比,SeNP的荧光信号更强。由于已知促炎激活的巨噬细胞会产生大量H 2 O 2,而发炎部位通常会引起与炎症相关的组织缺氧,因此SeNPs会被O 2进一步修饰。自给自足的光动力疗法功能。通过形成二硫键将过氧化氢酶固定在SeNPs上。细胞内二硫键的还原诱导过氧化氢酶的后续释放,从而催化H 2 O 2的分解。消耗H 2 O 2和产生O 2的光动力SeNP有效杀死活化的巨噬细胞,并猝灭与炎症有关的细胞内H 2 O 2和NO。SeNPs可能具有治疗纳米材料的潜力,可以成像并控制巨噬细胞的活化。
更新日期:2017-02-01
中文翻译:

H 2 O 2消耗和O 2生成硒纳米粒子的荧光成像和光动力治疗促炎性巨噬细胞。
巨噬细胞在慢性炎症疾病(CID)中起着关键作用,因此成像和控制活化的巨噬细胞对于检测和减少慢性炎症至关重要。在这项研究中,开发了具有光敏和巨噬细胞靶向双层的光动力学硒纳米颗粒(SeNPs)。光敏大分子的第一层由光敏剂(玫瑰红,RB)和硫醇化壳聚糖(壳聚糖-谷胱甘肽)的共轭物组成,导致等离子体耦合引起的红移和RB吸收谱带的扩展,吸收强度增加。电子顺磁共振(EPR)和二苯基蒽(DPA)淬灭研究表明,涂有光敏层的SeNP在产生单线态氧方面比单独的RB更有效(1O 2)在光照射下。通过使用乙二胺接头使透明质酸与叶酸缀合来合成活化的巨噬细胞可靶向大分子的第二层。促炎激活的巨噬细胞迅速内化了被靶向配体覆盖的SeNP,与未激活的巨噬细胞相比,SeNP的荧光信号更强。由于已知促炎激活的巨噬细胞会产生大量H 2 O 2,而发炎部位通常会引起与炎症相关的组织缺氧,因此SeNPs会被O 2进一步修饰。自给自足的光动力疗法功能。通过形成二硫键将过氧化氢酶固定在SeNPs上。细胞内二硫键的还原诱导过氧化氢酶的后续释放,从而催化H 2 O 2的分解。消耗H 2 O 2和产生O 2的光动力SeNP有效杀死活化的巨噬细胞,并猝灭与炎症有关的细胞内H 2 O 2和NO。SeNPs可能具有治疗纳米材料的潜力,可以成像并控制巨噬细胞的活化。













































 京公网安备 11010802027423号
京公网安备 11010802027423号