当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular determinant of the effects of hydrostatic pressure on protein folding stability.
Nature Communications ( IF 14.7 ) Pub Date : 2017-02-07 , DOI: 10.1038/ncomms14561
Calvin R. Chen , George I. Makhatadze
Nature Communications ( IF 14.7 ) Pub Date : 2017-02-07 , DOI: 10.1038/ncomms14561
Calvin R. Chen , George I. Makhatadze
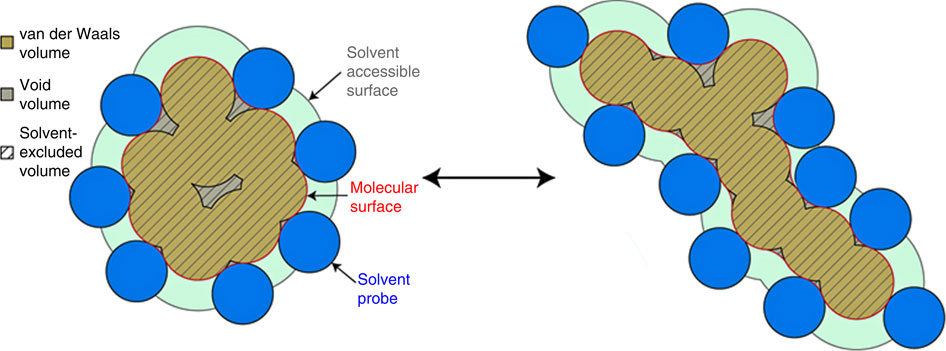
|
Hydrostatic pressure is an important environmental variable that plays an essential role in biological adaptation for many extremophilic organisms (for example, piezophiles). Increase in hydrostatic pressure, much like increase in temperature, perturbs the thermodynamic equilibrium between native and unfolded states of proteins. Experimentally, it has been observed that increase in hydrostatic pressure can both increase and decrease protein stability. These observations suggest that volume changes upon protein unfolding can be both positive and negative. The molecular details of this difference in sign of volume changes have been puzzling the field for the past 50 years. Here we present a comprehensive thermodynamic model that provides in-depth analysis of the contribution of various molecular determinants to the volume changes upon protein unfolding. Comparison with experimental data shows that the model allows quantitative predictions of volume changes upon protein unfolding, thus paving the way to proteome-wide computational comparison of proteins from different extremophilic organisms.
中文翻译:

静水压力对蛋白质折叠稳定性影响的分子决定因素。
静水压力是重要的环境变量,在许多极端微生物(例如耐压微生物)的生物适应中起着至关重要的作用。静水压力的增加,就像温度的增加一样,扰乱了蛋白质天然状态和未折叠状态之间的热力学平衡。实验上已经发现,静水压力的增加既可以增加也可以减少蛋白质的稳定性。这些观察结果表明,蛋白质解折叠后的体积变化既可以是正的,也可以是负的。在过去的50年中,这种体积变化迹象差异的分子细节一直困扰着该领域。在这里,我们提出了一个综合的热力学模型,该模型提供了对各种分子决定因素对蛋白质展开后体积变化的贡献的深入分析。与实验数据的比较表明,该模型可以定量预测蛋白质解折叠后的体积变化,从而为来自不同极端微生物的蛋白质的蛋白质组范围内的计算比较铺平了道路。
更新日期:2017-02-08
中文翻译:

静水压力对蛋白质折叠稳定性影响的分子决定因素。
静水压力是重要的环境变量,在许多极端微生物(例如耐压微生物)的生物适应中起着至关重要的作用。静水压力的增加,就像温度的增加一样,扰乱了蛋白质天然状态和未折叠状态之间的热力学平衡。实验上已经发现,静水压力的增加既可以增加也可以减少蛋白质的稳定性。这些观察结果表明,蛋白质解折叠后的体积变化既可以是正的,也可以是负的。在过去的50年中,这种体积变化迹象差异的分子细节一直困扰着该领域。在这里,我们提出了一个综合的热力学模型,该模型提供了对各种分子决定因素对蛋白质展开后体积变化的贡献的深入分析。与实验数据的比较表明,该模型可以定量预测蛋白质解折叠后的体积变化,从而为来自不同极端微生物的蛋白质的蛋白质组范围内的计算比较铺平了道路。







































 京公网安备 11010802027423号
京公网安备 11010802027423号