Synthesis ( IF 2.2 ) Pub Date : 2015-06-30 , DOI: 10.1055/s-0034-1380864 Rajender Karnekanti 1 , Marumamula Hanumaiah 1 , Gangavaram Sharma 1
|
Abstract
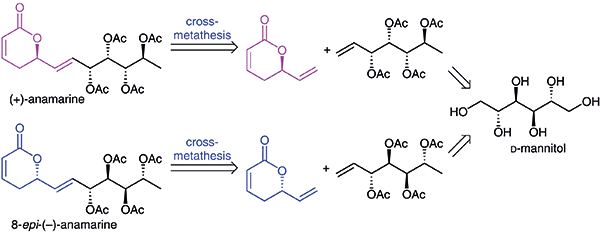
Stereoselective total synthesis of (+)-anamarine and the first synthesis of 8-epi-(–)-anamarine, its nonnatural diastereomer, were achieved from readily available d-mannitol. The key reactions involved were asymmetric dihydroxylation, cross-metathesis and ring-closing metathesis reactions. The approach is adoptable advantageously for the diversity-oriented synthesis of several related classes of natural products.
Stereoselective total synthesis of (+)-anamarine and the first synthesis of 8-epi-(–)-anamarine, its nonnatural diastereomer, were achieved from readily available d-mannitol. The key reactions involved were asymmetric dihydroxylation, cross-metathesis and ring-closing metathesis reactions. The approach is adoptable advantageously for the diversity-oriented synthesis of several related classes of natural products.
中文翻译:

d-甘露糖醇的立体选择性全合成(+)-鸟嘌呤和8-表-(-)-鸟嘌呤
摘要
立体选择性的全合成(+)-橘红和第一个合成的8- Epi -(-)-橘红是其非天然非对映异构体,是从现成的d-甘露醇中获得的。涉及的关键反应是不对称二羟基化,交叉复分解和闭环复分解反应。该方法有利地可用于几种相关类别的天然产物的面向多样性的合成。
立体选择性的全合成(+)-橘红和第一个合成的8- Epi -(-)-橘红是其非天然非对映异构体,是从现成的d-甘露醇中获得的。涉及的关键反应是不对称二羟基化,交叉复分解和闭环复分解反应。该方法有利地可用于几种相关类别的天然产物的面向多样性的合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号