当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal‐Free Dihydrogen Oxidation by a Borenium Cation: A Combined Electrochemical/Frustrated Lewis Pair Approach
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2014-07-18 , DOI: 10.1002/anie.201405721 Elliot J Lawrence 1 , Thomas J Herrington , Andrew E Ashley , Gregory G Wildgoose
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2014-07-18 , DOI: 10.1002/anie.201405721 Elliot J Lawrence 1 , Thomas J Herrington , Andrew E Ashley , Gregory G Wildgoose
Affiliation
|
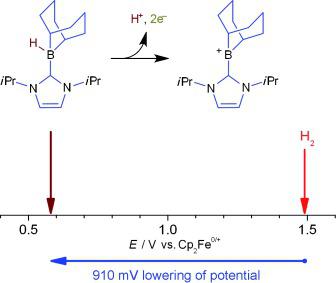
In order to use H2 as a clean source of electricity, prohibitively rare and expensive precious metal electrocatalysts, such as Pt, are often used to overcome the large oxidative voltage required to convert H2 into 2 H+ and 2 e−. Herein, we report a metal‐free approach to catalyze the oxidation of H2 by combining the ability of frustrated Lewis pairs (FLPs) to heterolytically cleave H2 with the in situ electrochemical oxidation of the resulting borohydride. The use of the NHC‐stabilized borenium cation [(IiPr2)(BC8H14)]+ (IiPr2=C3H2(NiPr)2, NHC=N‐heterocyclic carbene) as the Lewis acidic component of the FLP is shown to decrease the voltage required for H2 oxidation by 910 mV at inexpensive carbon electrodes, a significant energy saving equivalent to 175.6 kJ mol−1. The NHC–borenium Lewis acid also offers improved catalyst recyclability and chemical stability compared to B(C6F5)3, the paradigm Lewis acid originally used to pioneer our combined electrochemical/frustrated Lewis pair approach.
中文翻译:

硼阳离子的无金属二氢氧化:电化学/受阻路易斯对组合方法
为了使用H 2作为清洁电力来源,通常使用极其稀有且昂贵的贵金属电催化剂,例如Pt,来克服将H 2转化为2 H +和2 e -所需的大氧化电压。在此,我们报告了一种无金属催化 H 2氧化的方法,该方法将受挫路易斯对 (FLP) 异解裂解 H 2的能力与所得硼氢化物的原位电化学氧化相结合。使用NHC稳定的硼阳离子[(I i Pr 2 )(BC 8 H 14 )] + (I i Pr 2 =C 3 H 2 (N i Pr) 2 , NHC=N-杂环卡宾)作为FLP 的路易斯酸性组分可在廉价的碳电极上将 H 2氧化所需的电压降低 910 mV,相当于 175.6 kJ mol -1的显着节能。与 B(C 6 F 5 ) 3 相比,NHC-硼路易斯酸还提供了改进的催化剂可回收性和化学稳定性,B(C 6 F 5 ) 3是最初用于开创我们的组合电化学/受阻路易斯对方法的范例路易斯酸。
更新日期:2014-07-18
中文翻译:

硼阳离子的无金属二氢氧化:电化学/受阻路易斯对组合方法
为了使用H 2作为清洁电力来源,通常使用极其稀有且昂贵的贵金属电催化剂,例如Pt,来克服将H 2转化为2 H +和2 e -所需的大氧化电压。在此,我们报告了一种无金属催化 H 2氧化的方法,该方法将受挫路易斯对 (FLP) 异解裂解 H 2的能力与所得硼氢化物的原位电化学氧化相结合。使用NHC稳定的硼阳离子[(I i Pr 2 )(BC 8 H 14 )] + (I i Pr 2 =C 3 H 2 (N i Pr) 2 , NHC=N-杂环卡宾)作为FLP 的路易斯酸性组分可在廉价的碳电极上将 H 2氧化所需的电压降低 910 mV,相当于 175.6 kJ mol -1的显着节能。与 B(C 6 F 5 ) 3 相比,NHC-硼路易斯酸还提供了改进的催化剂可回收性和化学稳定性,B(C 6 F 5 ) 3是最初用于开创我们的组合电化学/受阻路易斯对方法的范例路易斯酸。































 京公网安备 11010802027423号
京公网安备 11010802027423号