当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polymerization of Ethylene by Silica‐Supported Dinuclear CrIII Sites through an Initiation Step Involving C?H Bond Activation
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2014-01-21 , DOI: 10.1002/anie.201308983 Matthew P. Conley , Murielle F. Delley , Georges Siddiqi , Giuseppe Lapadula , Sébastien Norsic , Vincent Monteil , Olga V. Safonova , Christophe Copéret
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2014-01-21 , DOI: 10.1002/anie.201308983 Matthew P. Conley , Murielle F. Delley , Georges Siddiqi , Giuseppe Lapadula , Sébastien Norsic , Vincent Monteil , Olga V. Safonova , Christophe Copéret
|
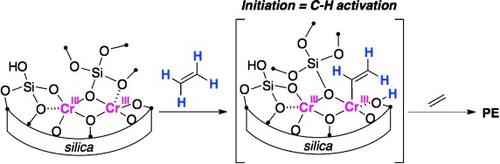
The insertion of an olefin into a preformed metal–carbon bond is a common mechanism for transition‐metal‐catalyzed olefin polymerization. However, in one important industrial catalyst, the Phillips catalyst, a metal–carbon bond is not present in the precatalyst. The Phillips catalyst, CrO3 dispersed on silica, polymerizes ethylene without an activator. Despite 60 years of intensive research, the active sites and the way the first CrC bond is formed remain unknown. We synthesized well‐defined dinuclear CrII and CrIII sites on silica. Whereas the CrII material was a poor polymerization catalyst, the CrIII material was active. Poisoning studies showed that about 65 % of the CrIII sites were active, a far higher proportion than typically observed for the Phillips catalyst. Examination of the spent catalyst and isotope labeling experiments showed the formation of a Si–(μ‐OH)–CrIII species, consistent with an initiation mechanism involving the heterolytic activation of ethylene at CrIIIO bonds.
中文翻译:

二氧化硅负载的双核CrIII位点通过涉及C的引发步骤聚合乙烯。H键活化
将烯烃插入到预先形成的金属碳键中是过渡金属催化的烯烃聚合的常见机制。但是,在一种重要的工业催化剂Phillips催化剂中,预催化剂中不存在金属-碳键。菲利普斯催化剂(分散在二氧化硅上的CrO 3)可在没有活化剂的情况下聚合乙烯。尽管进行了60年的深入研究,但尚不清楚活性部位和第一个CrC键形成的方式。我们在二氧化硅上合成了定义明确的双核Cr II和Cr III位点。Cr II材料是不良的聚合催化剂,而Cr III材料则具有活性。中毒研究表明,约65%的铬III位是活跃的,比通常在菲利普斯催化剂中观察到的比例高得多。用过的催化剂和同位素标记实验的检验表明一个SI-(μ-OH)-Cr的形成III物种,与涉及的乙烯在铬的异裂活化的引发机制是一致III O键。
更新日期:2014-01-21
中文翻译:

二氧化硅负载的双核CrIII位点通过涉及C的引发步骤聚合乙烯。H键活化
将烯烃插入到预先形成的金属碳键中是过渡金属催化的烯烃聚合的常见机制。但是,在一种重要的工业催化剂Phillips催化剂中,预催化剂中不存在金属-碳键。菲利普斯催化剂(分散在二氧化硅上的CrO 3)可在没有活化剂的情况下聚合乙烯。尽管进行了60年的深入研究,但尚不清楚活性部位和第一个CrC键形成的方式。我们在二氧化硅上合成了定义明确的双核Cr II和Cr III位点。Cr II材料是不良的聚合催化剂,而Cr III材料则具有活性。中毒研究表明,约65%的铬III位是活跃的,比通常在菲利普斯催化剂中观察到的比例高得多。用过的催化剂和同位素标记实验的检验表明一个SI-(μ-OH)-Cr的形成III物种,与涉及的乙烯在铬的异裂活化的引发机制是一致III O键。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号