当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Characterization of the Actinium Aquo Ion.
ACS Central Science ( IF 12.7 ) Pub Date : 2017-02-01 , DOI: 10.1021/acscentsci.6b00356 Maryline G Ferrier 1 , Benjamin W Stein 1 , Enrique R Batista 1 , John M Berg 1 , Eva R Birnbaum 1 , Jonathan W Engle 2 , Kevin D John 1 , Stosh A Kozimor 1 , Juan S Lezama Pacheco 3 , Lindsay N Redman 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-02-01 , DOI: 10.1021/acscentsci.6b00356 Maryline G Ferrier 1 , Benjamin W Stein 1 , Enrique R Batista 1 , John M Berg 1 , Eva R Birnbaum 1 , Jonathan W Engle 2 , Kevin D John 1 , Stosh A Kozimor 1 , Juan S Lezama Pacheco 3 , Lindsay N Redman 1
Affiliation
|
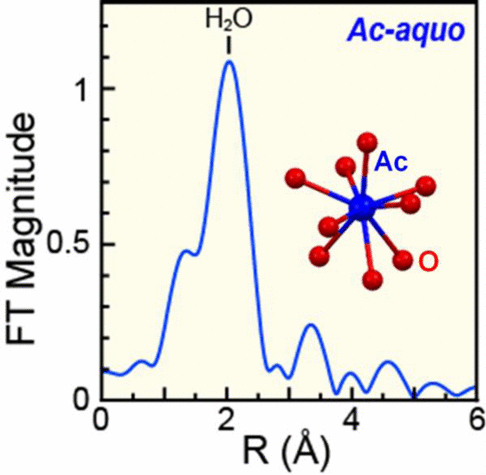
Metal aquo ions occupy central roles in all equilibria that define metal complexation in natural environments. These complexes are used to establish thermodynamic metrics (i.e., stability constants) for predicting metal binding, which are essential for defining critical parameters associated with aqueous speciation, metal chelation, in vivo transport, and so on. As such, establishing the fundamental chemistry of the actinium(III) aquo ion (Ac-aquo ion, Ac(H2O) x3+) is critical for current efforts to develop 225Ac [t1/2 = 10.0(1) d] as a targeted anticancer therapeutic agent. However, given the limited amount of actinium available for study and its high radioactivity, many aspects of actinium chemistry remain poorly defined. We overcame these challenges using the longer-lived 227Ac [t1/2 = 21.772(3) y] isotope and report the first characterization of this fundamentally important Ac-aquo coordination complex. Our X-ray absorption fine structure study revealed 10.9 ± 0.5 water molecules directly coordinated to the AcIII cation with an Ac-OH2O distance of 2.63(1) Å. This experimentally determined distance was consistent with molecular dynamics density functional theory results that showed (over the course of 8 ps) that AcIII was coordinated by 9 water molecules with Ac-OH2O distances ranging from 2.61 to 2.76 Å. The data is presented in the context of other actinide(III) and lanthanide(III) aquo ions characterized by XAFS and highlights the uniqueness of the large AcIII coordination numbers and long Ac-OH2O bond distances.
中文翻译:

in基水合离子的合成与表征。
在自然环境中定义金属络合的所有平衡中,金属水族离子均起着核心作用。这些络合物用于建立预测金属结合的热力学指标(即稳定性常数),这对于定义与水形态,金属螯合,体内运输等相关的关键参数至关重要。因此,建立act(III)quo离子(Ac-a离子,Ac(H2O)x3 +)的基本化学性质对于当前开发225Ac [t1 / 2 = 10.0(1)d]作为靶向抗癌药的工作至关重要治疗剂。然而,鉴于可用于研究的of的数量有限且其放射性很高,因此act化学的许多方面仍然定义不清。我们使用寿命更长的227Ac [t1 / 2 = 21]克服了这些挑战。772(3)y]同位素,并报告了这种根本重要的Ac-aquo配合物的首次表征。我们的X射线吸收精细结构研究显示,与AcIII阳离子直接配位的10.9±0.5个水分子的Ac-OH2O距离为2.63(1)Å。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。9±0.5个水分子直接与AcIII阳离子配位,Ac-OH2O距离为2.63(1)Å。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。9±0.5个水分子直接与AcIII阳离子配位,Ac-OH2O距离为2.63(1)Å。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。
更新日期:2017-02-01
中文翻译:

in基水合离子的合成与表征。
在自然环境中定义金属络合的所有平衡中,金属水族离子均起着核心作用。这些络合物用于建立预测金属结合的热力学指标(即稳定性常数),这对于定义与水形态,金属螯合,体内运输等相关的关键参数至关重要。因此,建立act(III)quo离子(Ac-a离子,Ac(H2O)x3 +)的基本化学性质对于当前开发225Ac [t1 / 2 = 10.0(1)d]作为靶向抗癌药的工作至关重要治疗剂。然而,鉴于可用于研究的of的数量有限且其放射性很高,因此act化学的许多方面仍然定义不清。我们使用寿命更长的227Ac [t1 / 2 = 21]克服了这些挑战。772(3)y]同位素,并报告了这种根本重要的Ac-aquo配合物的首次表征。我们的X射线吸收精细结构研究显示,与AcIII阳离子直接配位的10.9±0.5个水分子的Ac-OH2O距离为2.63(1)Å。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。9±0.5个水分子直接与AcIII阳离子配位,Ac-OH2O距离为2.63(1)Å。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。9±0.5个水分子直接与AcIII阳离子配位,Ac-OH2O距离为2.63(1)Å。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。该实验确定的距离与分子动力学密度泛函理论结果一致,该结果表明(在8 ps的过程中)AcIII由9个水分子(Ac-OH2O距离为2.61至2.76Å)协调。数据是在以XAFS为特征的其他act系元素(III)和镧系元素(III)水族离子的背景下给出的,并强调了较大的AcIII配位数和长的Ac-OH2O键距的独特性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号